Abstract
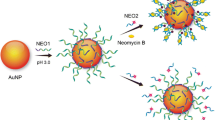
Based on a novel signal amplification strategy by catalytic hairpin assembly and displacement of G-quadruplex DNA, an enzyme-free, non-label fluorescent aptasensing approach was established for sensitive detection of four tetracycline veterinary drugs in milk. The network consisted of a pair of partially complementary DNA hairpins (HP1 and HP2). The DNA aptamer of four tetracycline veterinary drugs was located at the sticky end of the HP1. The ring region of HP1 rich in G and C could form a stable G-quadruplex structure, which could emit specific fluorescence signal after binding with the fluorescent dye and N-methylmesoporphyrin IX (NMM). When presented in the system, the target analytes would be repeatedly used to trigger a recycling procedure between the hairpins, generating numerous HP1–HP2 duplex complexes and displacing G-quadruplex DNA. Thus, the sensitive detection of target analytes was achieved in a wide linear range (0–1000 μg/L) with the detection limit of 4.6 μg/L. Moreover, this proposed method showed high discrimination efficiency towards target analytes against other common mismatched veterinary drugs, and could be successfully applied to the analysis of milk samples.

Schematic of target analyte detection based on catalytic hairpin assembly reaction and displacement of G-quadruplex





Similar content being viewed by others
References
Urkek B, Sengul M, Erkaya T, Aksakal V. Prevalence and comparing of some microbiological properties, somatic cell count and antibiotic residue of organic and conventional raw milk produced in Turkey. Korean J Food Sci An. 2017;37(2):264–73.
Chan D, Macarthur R, Fussell RJ, Wilford J, Budge G. Variability of residue concentrations of ciprofloxacin in honey from treated hives. Food Addit Contam A. 2017;34(4):552–61.
Liu X, Steele JC, Meng XZ. Usage, residue, and human health risk of antibiotics in Chinese aquaculture – a review. Environ Pollut (Barking, Essex: 1987). 2017;223:161–9.
Ahmed S, Ning J, Cheng G, Ahmad I, Li J, Mingyue L, et al. Receptor-based screening assays for the detection of antibiotics residues – a review. Talanta. 2017;166:176–86.
Uchida K, Konishi Y, Harada K, Okihashi M, Yamaguchi T, Do MH, et al. Monitoring of antibiotic residues in aquatic products in urban and rural areas of Vietnam. J Agr Food Chem. 2016;64(31):6133–8.
Olatoye IO, Daniel OF, Ishola SA. Screening of antibiotics and chemical analysis of penicillin residue in fresh milk and traditional dairy products in Oyo state, Nigeria. Vet World. 2016;9(9):948–54.
Moghadam MM, Amiri M, Riabi HR, Riabi HR. Evaluation of antibiotic residues in pasteurized and raw milk distributed in the south of Khorasan-e Razavi Province, Iran. J Clin Diagn Res. 2016;10(12):fc31–5.
Danaher M, Shanahan C, Butler F, Evans R, O'Sullivan D, Glynn D, et al. Risk-based approach to developing a national residue sampling plan for testing under European Union regulation for veterinary medicinal products and coccidiostat feed additives in domestic animal production. Food Addit Contam A. 2016;33(7):1155–65.
Qin J, Xie L, Ying Y. A high-sensitivity terahertz spectroscopy technology for tetracycline hydrochloride detection using metamaterials. Food Chem. 2016;211:300–5.
Cetinkaya F, Yibar A, Soyutemiz GE, Okutan B, Ozcan A, Karaca MY. Determination of tetracycline residues in chicken meat by liquid chromatography-tandem mass spectrometry. Food Addit Contam B. 2012;5(1):45–9.
Griffin MO, Fricovsky E, Ceballos G, Villarreal F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol-Cell Ph. 2010;299(3):C539–48.
Berendsen BJ, Van Rhijn JA. Residue analysis of tetracyclines in poultry muscle: shortcomings revealed by a proficiency test. Food Addit Contam. 2006;23(11):1141–8.
Wang Q, Lv Z, Tang Q, Gong CB, Lam MH, Ma XB, et al. Photoresponsive molecularly imprinted hydrogel casting membrane for the determination of trace tetracycline in milk. J Mol Recognit. 2016;29(3):123–30.
Hou J, Li H, Wang L, Zhang P, Zhou T, Ding H, et al. Rapid microwave-assisted synthesis of molecularly imprinted polymers on carbon quantum dots for fluorescent sensing of tetracycline in milk. Talanta. 2016;146:34–40.
Al-Bahry SN, Al-Mashani BM, Al-Ansari AS, Elshafie AE, Mahmoud IY. Escherichia coli tetracycline efflux determinants in relation to tetracycline residues in chicken. Asian Pac J Trop Med. 2013;6(9):718–22.
Wang S, Liu J, Yong W, Chen Q, Zhang L, Dong Y, et al. A direct competitive assay-based aptasensor for sensitive determination of tetracycline residue in honey. Talanta. 2015;131:562–9.
Wang S, Yong W, Liu J, Zhang L, Chen Q, Dong Y. Development of an indirect competitive assay-based aptasensor for highly sensitive detection of tetracycline residue in honey. Biosens Bioelectron. 2014;57:192–8.
Gao J, Wang H, Qu J, Wang H, Wang X. Development and optimization of a naphthoic acid-based ionic liquid as a "non-organic solvent microextraction" for the determination of tetracycline antibiotics in milk and chicken eggs. Food Chem. 2017;215:138–48.
Rodriguez MP, Pezza HR, Pezza L. Ultrasound-assisted dispersive liquid-liquid microextraction of tetracycline drugs from egg supplements before flow injection analysis coupled to a liquid waveguide capillary cell. Anal Bioanal Chem. 2016;408(22):6201–11.
Yang X, Zhang S, Yu W, Liu Z, Lei L, Li N, et al. Ionic liquid-anionic surfactant based aqueous two-phase extraction for determination of antibiotics in honey by high-performance liquid chromatography. Talanta. 2014;124:1–6.
Du F, Zheng X, Sun L, Qin Q, Guo L, Ruan G. Development and validation of polymerized high internal phase emulsion monoliths coupled with HPLC and fluorescence detection for the determination of trace tetracycline antibiotics in environmental water samples. J Sep Sci. 2015;38(21):3774–80.
Jiao Z, Zhang S, Chen H. Determination of tetracycline antibiotics in fatty food samples by selective pressurized liquid extraction coupled with high-performance liquid chromatography and tandem mass spectrometry. J Sep Sci. 2015;38(1):115–20.
Deng B, Xu Q, Lu H, Ye L, Wang Y. Pharmacokinetics and residues of tetracycline in crucian carp muscle using capillary electrophoresis on-line coupled with electrochemiluminescence detection. Food Chem. 2012;134(4):2350–4.
Wu P, Hou X, Xu JJ, Chen HY. Ratiometric fluorescence, electrochemiluminescence, and photoelectrochemical chemo/biosensing based on semiconductor quantum dots. Nanoscale. 2016;8(16):8427–42.
Raspor Lainscek P, Biasizzo M, Henigman U, Dolenc J, Kirbis A. Implementation of the Bacillus cereus microbiological plate used for the screening of tetracyclines in raw milk samples with STAR protocol – the problem with false-negative results solved. Food Addit Contam A. 2014;31(11):1840–9.
Song E, Yu M, Wang Y, Hu W, Cheng D, Swihart MT, et al. Multi-color quantum dot-based fluorescence immunoassay array for simultaneous visual detection of multiple antibiotic residues in milk. Biosens Bioelectron. 2015;72:320–5.
Aga DS, O'Connor S, Ensley S, Payero JO, Snow D, Tarkalson D. Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography-mass spectrometry. J Agri Food Chem. 2005;53(18):7165–71.
Aslipashaki SN, Khayamian T, Hashemian Z. Aptamer based extraction followed by electrospray ionization-ion mobility spectrometry for analysis of tetracycline in biological fluids. J Chromatogr B. 2013;925:26–32.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science (New York, NY). 1990;249(4968):505–10.
Famulok M, Mayer G. Aptamer modules as sensors and detectors. Accounts Chem Res. 2011;44(12):1349–58.
Ma H, Liu J, Ali MM, Mahmood MA, Labanieh L, Lu M, et al. Nucleic acid aptamers in cancer research, diagnosis, and therapy. Chem Soc Rev. 2015;44(5):1240–56.
Huang S, Gan N, Liu H, Zhou Y, Chen Y, Cao Y. Simultaneous and specific enrichment of several amphenicol antibiotics residues in food based on novel aptamer functionalized magnetic adsorbents using HPLC-DAD. J Chromatogr B. 2017;1060:247–54.
Zhou W, Saran R, Liu J. Metal Sensing by DNA. Chem Rev. 2017;117(12):8272–325.
Mercier MC, Dontenwill M, Choulier L. Selection of nucleic acid aptamers targeting tumor cell-surface protein biomarkers. Cancers (Basel). 2017;9(6). https://doi.org/10.3390/cancers9060069.
Bayrac C, Eyidogan F, Avni Oktem H. DNA aptamer-based colorimetric detection platform for Salmonella enteritidis. Biosens Bioelectron. 2017;98:22–8.
Song MS, Sekhon SS, Shin WR, Kim HC, Min J, Ahn JY, et al. Detecting and discriminating Shigella sonnei using an aptamer-based fluorescent biosensor platform. Molecules. 2017;22(5). https://doi.org/10.3390/molecules22050825.
Nilaratanakul V, Hauer DA, Griffin DE. Development and characterization of Sindbis virus with encoded fluorescent RNA aptamer Spinach2 for imaging of replication and immune-mediated changes in intracellular viral RNA. J Gen Virol. 2017;98(5):992–1003.
Li Y, Wang R. Aptasensors for detection of avian influenza virus H5N1. Methods Mol Biol. 2017;1572:379–402.
Luo S, Wang S, Luo N, Hu FCC, Zhang K. The application of aptamer 5TR1 in triple negative breast cancer target therapy. J Cellular Biochem. 2017; https://doi.org/10.1002/jcb.26254.
Park JH, Byun JY, Jang H, Hong D, Kim MG. A highly sensitive and widely adaptable plasmonic aptasensor using berberine for small-molecule detection. Biosens Bioelectron. 2017;97:292–8.
Alsager OA, Kumar S, Hodgkiss JM. Lateral flow aptasensor for small molecule targets exploiting adsorption and desorption interactions on gold nanoparticles. Anal Chem. 2017; https://doi.org/10.1021/acs.analchem.7b00906.
Ramezani M, Mohammad Danesh N, Lavaee P, Abnous K, Mohammad Taghdisi S. A novel colorimetric triple-helix molecular switch aptasensor for ultrasensitive detection of tetracycline. Biosens Bioelectron. 2015;70:181–7.
Kwon YS, Ahmad Raston NH, Gu MB. An ultrasensitive colorimetric detection of tetracyclines using the shortest aptamer with highly enhanced affinity. Chem Commun. 2014;50(1):40–2.
Chen Z, Chengjun S, Zewei L, Kunping L, Xijian Y, Haimin Z, et al. Fiber optic biosensor for detection of genetically modified food based on catalytic hairpin assembly reaction and nanocomposites assisted signal amplification. Sensor Actuat B–Chem. 2018;254:956–65.
Yin P, Choi HM, Calvert CR, Pierce NA. Programming biomolecular self-assembly pathways. Nature. 2008;451(7176):318–22.
Sun XY, Zhao P, Jin SF, Liu MC, Wang XH, Huang YM, et al. Shedding lights on the flexible-armed porphyrins: human telomeric G4 DNA interaction and cell photocytotoxicity research. J Photochem Photobio B. 2017;173:606–17.
Ambrus A, Chen D, Dai J, Bialis T, Jones RA, Yang D. Human telomeric sequence forms a hybrid-type intramolecular G-quadruplex structure with mixed parallel/antiparallel strands in potassium solution. Nucleic Acids Res. 2006;34(9):2723–35.
Flaugnatti N, Journet L. Identification of effectors: precipitation of supernatant material. Methods Mol Biol. 2017;1615:459–64.
Hee KH, Leaw YKJ, Ong JL, Lee LS. Development and validation of liquid chromatography tandem mass spectrometry method quantitative determination of polymyxin B1, polymyxin B2, polymyxin B3, and isoleucine-polymyxin B1 in human plasma and its application in clinical studies. J Pharmaceut Biomed. 2017;140:91–7.
Acknowledgments
The authors appreciate funding from China Postdoctoral Science Foundation (2016M590894) and the Fundamental Research Funds for the Central Universities (2012017yjsy207).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest to declare.
Electronic supplementary material
ESM 1
(PDF 142 kb)
Rights and permissions
About this article
Cite this article
Zhou, C., Zou, H., Sun, C. et al. Fluorescent aptasensor for detection of four tetracycline veterinary drugs in milk based on catalytic hairpin assembly reaction and displacement of G-quadruplex. Anal Bioanal Chem 410, 2981–2989 (2018). https://doi.org/10.1007/s00216-018-0981-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0981-2