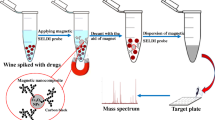
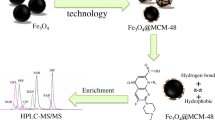
Abstract
In this study, the strategy of unique adsorbent combined with isotope labeled internal standards was used to significantly reduce the matrix effect for the enrichment and analysis of nine fluoroquinolones in a complex sample by liquid chromatography coupled to quadrupole linear ion trap mass spectrometry (LC-QqQLIT-MS/MS). The adsorbent was prepared conveniently by functionalizing Fe3O4@SiO2 microspheres with phenyl and tetrazolyl groups, which could adsorb fluoroquinolones selectively via hydrophobic, electrostatic, and π–π interactions. The established magnetic solid-phase extraction (MSPE) method as well as using stable isotope labeled internal standards in the next MS/MS detection was able to reduce the matrix effect significantly. In the process of LC-QqQLIT-MS/MS analysis, the precursor and product ions of the analytes were monitored quantitatively and qualitatively on a QTrap system equipped simultaneously with the multiple reaction monitoring (MRM) and enhanced product ion (EPI) scan. Subsequently, the enrichment method combined with LC-QqQLIT-MS/MS demonstrated good analytical features in terms of linearity (7.5–100.0 ng mL-1, r > 0.9960), satisfactory recoveries (88.6%–118.3%) with RSDs < 12.0%, LODs = 0.5 μg kg-1 and LOQs = 1.5 μg kg-1 for all tested analytes. Finally, the developed MSPE-LC-QqQLIT-MS/MS method had been successfully applied to real pork samples for food-safety risk monitoring in Ningxia Province, China.

Mechanism of reducing matrix effect through the as-prepared adsorbent.







Similar content being viewed by others
References
Carbone M, Pennisi MG, Masucci M, De Sarro A, Giannone M, Fera MT. Activity and postantibiotic effect of marbofloxacin, enrofloxacin, difloxacin and ciprofloxacin against feline Bordetella bronchiseptica isolates. Vet Microbiol. 2001;81:79–84.
Van Bambeke F, Michot JM, Van Eldere J, Tulkens PM. Quinolones in 2005: an update. Clin Microbiol Infect. 2005;11:256–80.
Hamad B. The antibiotics market. Nat Rev Drug Discov. 2010;9:675–6.
Lipsky BA, Baker CA. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin Infect Dis. 1999;28:352–64.
Owens RJ, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41:144–57.
Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72:381–93.
Announcement No. 235 of Ministry of Agriculture is the national standards for maximum residue level of veterinary drugs in food of animal origin (2002) Ministry of Agriculture of People's Republic of China. Available at http://www.moa.gov.cn/zwllm/tzgg/gg/200302/t20030226_59300.htm. Accessed 7 Dec 2017
Commission regulation (EU) (2010) No. 37/2010 of 22nd December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J L 15
Rambla-Alegre M, Collado-Sanchez MA, Esteve-Romero J, Carda-Broch S. Quinolones control in milk and eggs samples by liquid chromatography using a surfactant-mediated mobile phase. Anal Bioanal Chem. 2011;400:1303–13.
Lombardo-Aguei M, Gamiz-Gracia L, Cruces-Blanco C, Garcia-Campana AM. Comparison of different sample treatments for the analysis of quinolones in milk by capillary-liquid chromatography with laser induced fluorescence detection. J Chromatogr A. 2011;1218:4966–71.
Tsai W, Chuang H, Chen H, Huang J, Chen H, Cheng S, et al. Application of dispersive liquid–liquid microextraction and dispersive micro-solid-phase extraction for the determination of quinolones in swine muscle by high-performance liquid chromatography with diode-array detection. Anal Chim Acta. 2009;656:56–62.
Karbiwnyk CM, Carr LE, Turnipseed SB, Andersen WC, Miller KE. Determination of quinolone residues in shrimp using liquid chromatography with fluorescence detection and residue confirmation by mass spectrometry. Anal Chim Acta. 2007;596:257–63.
Hermo MP, Nemutlu E, Kir S, Barron D, Barbosa J. Improved determination of quinolones in milk at their MRL levels using LC-UV, LC-FD, LC-MS, and LC-MS/MS and validation in line with regulation 2002/657/EC. Anal Chim Acta. 2008;613:98–107.
Galarini R, Fioroni L, Angelucci F, Tovo GR, Cristofani E. Simultaneous determination of eleven quinolones in animal feed by liquid chromatography with fluorescence and ultraviolet absorbance detection. J Chromatogr A. 2009;1216:8158–64.
Evaggelopoulou EN, Samanidou VF. HPLC confirmatory method development for the determination of seven quinolones in salmon tissue (Salmo salar L.) validated according to the European Union Decision 2002/657/EC. Food Chem. 2013;136:479–84.
Hoof NV, Wasch KD, Okerman L, Reybroeck W, Poelmans S, Noppe H, et al. Validation of a liquid chromatography-tandem mass spectrometric method for the quantification of eight quinolones in bovine muscle, milk and aqua-cultured products. Anal Chim Acta. 2005;529:265–72.
Xu H, Wang T, Zhao Q, Zeng Q, Wang H, Xu Y, et al. Analysis of fluoroquinolones in animal feed based on microwave-assisted extraction by LC-MS-MS determination. Chromatographia. 2011;74:267–74.
Lee H, Peart TE, Svoboda ML. Determination of ofloxacin, norfloxacin, and ciprofloxacin in sewage by selective solid-phase extraction, liquid chromatography with fluorescence detection, and liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2007;1139:45–52.
Lara FJ, Garcia-Campana AM, Ales-Barrero F, Bosque-Sendra JM, Garcia-Ayuso LE. Multiresidue method for the determination of quinolone antibiotics in bovine raw milk by capillary electrophoresis-tandem mass spectrometry. Anal Chem. 2006;78:7665–73.
Tajabadi F, Ghambarian M, Yamini Y, Yazdanfar N. Combination of hollow fiber liquid phase microextraction followed by HPLC-DAD and multivariate curve resolution to determine antibacterial residues in foods of animal origin. Talanta. 2016;160:400–9.
Poliwoda A, Krzyzak M, Wieczorek PP. Supported liquid membrane extraction with single hollow fiber for the analysis of fluoroquinolones from environmental surface water samples. J Chromatogr A. 2010;1217:3590–7.
Castillo-Garcia ML, Aguilar-Caballos MP, Gomez-Hens A. A europium- and terbium-coated magnetic nanocomposite as sorbent in dispersive solid phase extraction coupled with ultra-high performance liquid chromatography for antibiotic determination in meat samples. J Chromatogr A. 2015;1425:73–80.
Zheng H, Mo J, Zhang Y, Gao Q, Ding J, Yu Q, et al. Facile synthesis of magnetic molecularly imprinted polymers and its application in magnetic solid phase extraction for fluoroquinolones in milk samples. J Chromatogr A. 2014;1329:17–23.
He H, Dong C, Li B, Dong J, Bo T, Wang T, et al. Fabrication of enrofloxacin imprinted organic-inorganic hybrid mesoporous sorbent from nanomagnetic polyhedral oligomeric silsesquioxanes for the selective extraction of fluoroquinolones in milk samples. J Chromatogr A. 2014;1361:23–33.
Wang H, Liu Y, Wei S, Yao S, Zhang J, Huang H. Selective extraction and determination of fluoroquinolones in bovine milk samples with montmorillonite magnetic molecularly imprinted polymers and capillary electrophoresis. Anal Bioanal Chem. 2016;408:589–98.
Manbohi A, Hamid Ahmad S. In-tube magnetic solid phase microextraction of some fluoroquinolones based on the use of sodium dodecyl sulfate coated Fe3O4 nanoparticles packed tube. Anal Chim Acta. 2015;885:114–21.
Jimenez V, Companyo R, Guiteras J. Validation of a method for the analysis of nine quinolones in eggs by pressurized liquid extraction and liquid chromatography with fluorescence detection. Talanta. 2011;85:596–606.
Zhu W, Yang J, Wang Z, Wang C, Liu Y, Zhang L. Rapid determination of 88 veterinary drug residues in milk using automated TurborFlow online clean-up mode coupled to liquid chromatography-tandem mass spectrometry. Talanta. 2016;148:401–11.
Prutthiwanasan B, Phechkrajang C, Suntornsuk L. Fluorescent labelling of ciprofloxacin and norfloxacin and its application for residues analysis in surface water. Talanta. 2016;159:74–9.
Alcaraz MR, Culzoni MJ, Goicoechea HC. Enhanced fluorescence sensitivity by coupling yttrium–analyte complexes and three-way fast high-performance liquid chromatography data modeling. Anal Chim Acta. 2016;902:50–8.
Lombardo-Agui M, Garcia-Campana AM, Gamiz-Gracia L, Cruces-Blanco C. Determination of quinolones of veterinary use in bee products by ultra-high performance liquid chromatography-tandem mass spectrometry using a QuEChERS extraction procedure. Talanta. 2012;93:193–9.
Dorival-Garcia N, Labajo-Recio C, Zafra-Gomez A, Juarez-Jimenez B, Vilchez J. Improved sample treatment for the determination of 17 strong sorbed quinolone antibiotics from compost by ultrahigh performance liquid chromatography tandem mass spectrometry. Talanta. 2015;138:247–57.
Guidi LR, Santos FA, Ribeiro AC, Fernandes C, Silva LH, Gloria MB. A simple, fast, and sensitive screening LC-ESI-MS/MS method for antibiotics in fish. Talanta. 2017;163:85–93.
Hager JW, Le Blanc JCY. Product ion scanning using a Q-q-Qlinear ion trap (Q TRAP) mass spectrometer. Rapid Commun Mass Spectrom. 2003;17:1056–64.
Wen B, Ma L, Nelson SD, Zhu M. High-throughput screening and characterization of reactive metabolites using polarity switching of hybrid triple quadrupole linear ion trap mass spectrometry. Anal Chem. 2008;80:1788–99.
Kong W, Wen J, Yang Y, Qiu F, Sheng P, Yang M. Simultaneous targeted analysis of five active compounds in licorice by ultra-fast liquid chromatography coupled to hybrid linear-ion trap tandem mass spectrometry. Analyst. 2014;139:1883–94.
Huang C, Guo B, Wang X, Li J, Zhu W, Chen B, et al. A generic approach for expanding homolog-targeted residue screening of sulfonamides using a fast matrix separation and class-specific fragmentation-dependent acquisition with a hybrid quadrupole-linear ion trap mass spectrometer. Anal Chim Acta. 2012;737:83–98.
Xing Y, Meng W, Sun W, Li D, Yu Z, Tong L, et al. Simultaneous qualitative and quantitative analysis of 21 mycotoxins in Radix Paeoniae Alba by ultra-high performance liquid chromatography quadrupole linear ion trap mass spectrometry and QuEChERS for sample preparation. J Chromatogr B. 2016;1031:202–13.
Liu X, Yu Y, Li Y, Zhang H, Ling J, Sun X, et al. Fluorocarbon-bonded magnetic mesoporous microspheres for the analysis of perfluorinated compounds in human serum by high-performance liquid chromatography coupled to tandem mass spectrometry. Anal Chim Acta. 2014;844:35–43.
Rubert J, Soler C, Manes J. Evaluation of matrix solid-phase dispersion (MSPD) extraction for multi-mycotoxin determination in different flours using LC-MS/MS. Talanta. 2011;85:206–15.
Liu J, Sun Z, Deng Y, Zou Y, Li C, Guo X, et al. Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew Chem Int Ed. 2009;48:5875–9.
Meng J, Bu J, Deng C, Zhang X. Preparation of polypyrrole-coated magnetic particles for micro solid-phase extraction of phthalates in water by gas chromatography-mass spectrometry analysis. J Chromatogr A. 2011;1218:1585–91.
Tartaj P, Serna CJ. Synthesis of monodisperse superparamagnetic Fe/Silica nan spherical composites. J Am Chem Soc. 2003;125:15754–5.
Long Y, Chen Y, Yang F, Chen C, Pan D, Cai Q, et al. Triphenylamine-functionalized magnetic microparticles as a new adsorbent coupled with high performance liquid chromatography for the analysis of trace polycyclic aromatic hydrocarbons in aqueous samples. Analyst. 2012;137:2716–22.
European Commission Decision 2002/657/EC. Implementing Council Direc-tive96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Commun. 2002;L221:8–36.
Zhang Y, Chen Y, Wang C, Wei Y. Immobilization of 5-aminopyridine-2-tetrazole on cross-linked polystyrene for the preparation of a new adsorbent to remove heavy metal ions from aqueous solution. J Hazard Mater. 2014;276:129–37.
Lei G, Xiong X, Wei Y, Zheng X, Zheng J. Novel tetrazole-functionalized ion exchanger for weak cation-exchange chromatography of proteins. J Chromatogr A. 2011;1187:197–204.
Ma Z, Guan Y, Liu H. Synthesis and characterization of micron-sized monodisperse superparamagnetic polymer particles with amino groups. J Polym Sci Part A. 2005;43:3433–9.
Frenich AG, Romero-Gonzalez R, Gomez-Perez ML, Vidal JLM. Multi-mycotoxin analysis in eggs using a QuEChERS-based extraction procedure and ultra-high-pressure liquid chromatography coupled to triple quadrupole mass spectrometry. J Chromatogr A. 2011;1218:4349–56.
Antignac J, De Wasch K, Monteau F, De Brabander H, Andre F, Le Bizec B. The ion suppression phenomenon in liquid chromatography-mass spectrometry and its consequences in the field of residue analysis. Anal Chim Acta. 2005;529:129–36.
Eurachem/CITAC Guide: Quantifying Uncertainty in Analytical Measurement, 3rd ed., 2012, 978-0-948926-30-3. Available at https://www.eurachem.org/index.php/publications/guides/quam#translations. Accessed 7 Dec 2017
Meyer VR. Measurement uncertainty. J Chromatogr A. 2007;1158:15–24.
Pindado JO, Perez Pastor RM. Estimation of measurement uncertainty of pesticides, polychlorinated biphenyls and polyaromatic hydrocarbons in sediments by using gas chromatography-mass spectrometry. Anal Chim Acta. 2012;724:20–9.
National Standard of the People’s Republic of China, GB/T 21312-2007 (2007) Analysis of 14 quinolones in food of animal origin by high performance liquid chromatography tandem mass spectrometry
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 21475104, 21775121, and 21575114), the Industry Development Project by the Science and Technology Department of Shaanxi Province (no. 2016GY-214), and the Ningxia Provincial National Natural Science Foundation (no. NZ14226).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 572 kb)
Rights and permissions
About this article
Cite this article
Xu, F., Liu, F., Wang, C. et al. Use of phenyl/tetrazolyl-functionalized magnetic microspheres and stable isotope labeled internal standards for significant reduction of matrix effect in determination of nine fluoroquinolones by liquid chromatography-quadrupole linear ion trap mass spectrometry. Anal Bioanal Chem 410, 1709–1724 (2018). https://doi.org/10.1007/s00216-017-0821-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0821-9