Abstract
An aptamer-based biosensor was developed for the detection of doxorubicin using electrochemical impedance spectroscopy. Doxorubicin and its 14-dehydroxylated version daunorubicin are anthracyclines often used in cancer treatment. Due to their mutagenic and cardiotoxic effects, detection in groundwater is desirable. We developed a biosensor using the daunorubicin-binding aptamer as biological recognition element. The aptamer was successfully co-immobilized with mercaptohexanol on gold and a density of 1.3*1013 ± 2.4*1012 aptamer molecules per cm2 was achieved. The binding of doxorubicin to the immobilized aptamer was detected by electrochemical impedance spectroscopy. The principle is based on the inhibition of electron transfer between electrode and ferro-/ferricyanide in solution caused by the binding of doxorubicin to the immobilized aptamer. A linear relationship between the charge transfer resistance (R ct ) and the doxorubicin concentration was obtained over the range of 31 nM to 125 nM doxorubicin, with an apparent binding constant of 64 nM and a detection limit of 28 nM. With the advantages of high sensitivity, selectivity, and simple sensor construction, this method shows a high potential of impedimetric aptasensors in environmental monitoring.

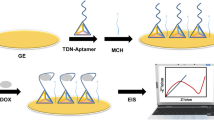
Measurement chamber and immobilization principle for the detection of doxorubicin by electrochemical impedance spectroscopy.





Similar content being viewed by others
References
Rahman A, Goodman A, Foo W, Harvey J, Smith FP, Schein PS. Clinical pharmacology of daunorubicin in Phase I patients with solid tumors: development of an analytical methodology for daunorubicin and its metabolites. Semin Oncol. 1984;11(4):36–44.
de los Santos-Alvarez N, Lobo-Castanon MJ, Miranda-Ordieres AJ, Tunon-Blanco P (2008) Aptamers as recognition elements for label-free analytical devices. TrAC Trends Anal Chem 27(5):437-446
Hanna AD, Lam A, Tham S, Dulhunty AF, Beard NA. Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Mol Pharmacol. 2014;86(4):438–49.
Mahnik SN, Lenz K, Weissenbacher N, Mader RM, Fuerhacker M. Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere. 2007;66, 30(1):–37.
Chisvert A, Sisternes J, Balaguer Á, Salvador A. A gas chromatography–mass spectrometric method to determine skin-whitening agents in cosmetic products. Talanta. 2010;81(1):530–6.
Diallo S, Lecanu L, Greeson J, Papadopoulos V. A capillary gas chromatography/mass spectrometric method for the quantification of hydroxysteroids in human plasma. Analytical Biochemistry. 2004;324(1):123–30.
Kaushik D, Bansal G. Characterization of degradation products of idarubicin through LC-UV, MSn, and LC-MS-TOF studies. J Pharmaceut Biomedi Anal. 2013;85:123–31.
Fogli S, Danesi R, Innocenti F, Di Paolo A, Bocci G, Barbara C, Del Tacca M. An improved HPLC method for therapeutic drug monitoring of daunorubicin, idarubicin, doxorubicin, epirubicin, and their 13-dihydro metabolites in human plasma. Ther Drug Monit. 1999;21(3):367–75.
Chin DL, Lum BL, Sikic BI. Rapid determination of PEGylated liposomal doxorubicin and its major metabolite in human plasma by ultraviolet-visible high-performance liquid chromatography. J Chromatogr B Anal Technol Biomedi Life Sci. 2002;779(2):259–69.
Hassan HNA, Barsoum BN, Habib IHI. Simultaneous spectrophotometric determination of rutin, quercetin, and ascorbic acid in drugs using a Kalman filter approach. J Pharmaceut Biomed Anal. 1999;20(1):315–20.
Zhang F, Du Y, Ye B, Li P. Study on the interaction between the chiral drug of propranolol and α1-acid glycoprotein by fluorescence spectrophotometry. J Photochem Photobiol B Biology. 2007;86(3):246–51.
Sun S, Huang X, Ma M, Qiu N, Cai Z, Luo Z, Alies NP. Systematic evaluation of avidin–biotin interaction by fluorescence spectrophotometry. Spectrochim Acta Part A Mol Biomol Spectrosc. 2012;89:99–104.
Yang X, Gao H, Qian F, Zhao C, Liao X. Internal standard method for the measurement of doxorubicin and daunorubicin by capillary electrophoresis with in-column double optical-fiber LED-induced fluorescence detection. J Parmaceut Biomed Anal. 2016;117:118–24.
Simeon N, Chatelut E, Canal P, Nertz M, Couderc F. Anthracycline analysis by capillary electrophoresis. Application to the analysis of daunorubicine in Kaposi sarcoma tumor. J Chromatogr A. 1999;853(1/2):449–54.
Erdem A, Karadeniz H, Caliskan A. Dendrimer modified graphite sensors for detection of anticancer drug daunorubicin by voltammetry and electrochemical impedance spectroscopy. Analyst. 2011;136(5):1041–5.
Strehlitz B, Reinemann C, Linkorn S, Stoltenburg R. Aptamers for pharmaceuticals and their application in environmental analytics. Bioanal Rev. 2012;4(1):1–30.
Velasco-Garcia MN, Missailidis S. New trends in aptamer-based electrochemical biosensors. Gene Ther Mol Biol. 2009;13(A):1–9.
Mann D, Reinemann C, Stoltenburg R, Strehlitz B. In vitro selection of DNA aptamers binding ethanolamine. Biochem Biophys Res Commun. 2005;338(4):1928–34.
Liang G, Man Y, Jin X, Pan L, Liu X. Aptamer-based biosensor for label-free detection of ethanolamine by electrochemical impedance spectroscopy. Anal Chim Acta. 2016;36:222–8.
Baker BR, Lai RY, Wood MS, Doctor EH, Heeger AJ, Plaxco KW. An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J Am Chem Soc. 2006;128(10):3138–9.
Schoukroun-Barnes LR, Wagan S, White RJ. Enhancing the analytical performance of electrochemical RNA aptamer-based sensors for sensitive detection of aminoglycoside antibiotics. Anal Chem. 2014;86(2):1131–7.
White RJ, Rowe AA, Plaxco KW. Re-engineering aptamers to support reagentless, self-reporting electrochemical sensors. Analyst. 2010;135(3):589–94.
Stojanovic MN, de Prada P, Landry DW. Aptamer-based folding fluorescent sensor for cocaine. J Am Chem Soc. 2001;123(21):4928–31.
Kim YS, Jung HS, Matsuura T, Lee HY, Kawai T, Gu MB. Electrochemical detection of 17β-estradiol using DNA aptamer immobilized gold electrode chip. Biosens Bioelectron. 2007;22(11):2525–31.
Wochner A, Menger M, Orgel D, Cech B, Rimmele M, Erdmann VA, Glökler J. A DNA aptamer with high affinity and specificity for therapeutic anthracyclines. Anal Biochem. 2008;373(1):34–42.
Sauerbrey G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Zeitschrift Physik. 1959;155(2):206–22.
Oesch U, Janata J. Electrochemical study of gold electrodes with anodic oxide films, I. Formation and reduction behaviour of anodic oxides on gold. Electrochim Acta. 1983;28(9):1237–46.
Keighley SD, Li P, Estrela P, Migliorato P. Optimization of DNA immobilization on gold electrodes for label-free detection by electrochemical impedance spectroscopy. Biosens Bioelectron. 2008;23(8):1291–7.
Hirschorn B, Orazem ME, Tribollet B, Vivier V, Frateur I, Musiani M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim Acta. 2010;55(21):6218–27.
Ilgu M, Nilsen-Hamilton M. Aptamers in analytics. Analyst. 2016;141(5):1551–68. https://doi.org/10.1039/c5an01824b .
Frense D, Kang S, Schieke K, Reich P, Barthel A, Pliquett U, Nacke T, Brian C, Beckmann D. Label-free impedimetric biosensor for thrombin using the thrombin-binding aptamer as receptor. J Phys: Conference Series. 2013;434:012091.
Li X, Shen L, Zhang D, Qi H, Gao Q, Ma F, Zhang C. Electrochemical impedance spectroscopy for study of aptamer–thrombin interfacial interactions. Biosens Bioelectron. 2008;23(11):1624–30.
Yang H, Ji J, Liu Y, Kong JL, Liu BH. An aptamer-based biosensor for sensitive thrombin detection. Electrochem Commun. 2009;11(1):38–40.
Yao CY, Qi YZ, Zhao YH, Xiang Y, Chen QH, Fu WL. Aptamer-based piezoelectric quartz crystal microbalance biosensor array for the quantification of IgE. Biosens Bioelectron. 2009;24(8):2499–503.
Papamichael KI, Kreuzer MP, Guilbault GG. Viability of allergy (IgE) detection using an alternative aptamer receptor and electrochemical means. Sensors Actuators B Chemical. 2007;121(1):178–86.
Ohuchi S, Mori Y, Nakamura Y. Evolution of an inhibitory RNA aptamer against T7 RNA polymerase. FEBS Open Bio. 2012;2:203–7.
Hynek D, Krejcova L, Zitka O, Adam V, Trnkova L, Sochor J. Electrochemical study of doxorubicin interaction with different sequences of single stranded oligonucleotides. Part I. Int J Electrochem Sc. 2012;7(1):13–33.
Gui-Fang C, Jie Z, Yong-Hua T, Pin-Gang H, Yu-Zhi F, Stiborová M, Eckschlager T, Hubálek J, Kizek R. Study on the interaction between antitumor drug daunomycin and DNA. Chin J Chem. 2005;23(5):576–80.
Cattoni DI, Chara O, Kaufman SB, González Flecha FL. Cooperativity in binding processes: new insights from phenomenological modeling. PLOS ONE. 2016;10(12):e0146043.
Swain MD, Octain J, Benson DE. Unimolecular, soluble semiconductor nanoparticle-based biosensors for thrombin using charge/electron transfer. Bioconj Chem. 2008;19(12):2520–6.
Urmann K, Reich P, Walter JG, Beckmann D, Segal E, Scheper T. Rapid and label-free detection of protein a by aptamer-tethered porous silicon nanostructures. J Biotechnol. 2017;257:171–7.
Blouin S, Lafontaine DA. A loop–loop interaction and a K-turn motif located in the lysine aptamer domain are important for the riboswitch gene regulation control. RNA. 2007;13(8):1256–67.
Shi P, Zhang Y, Yu Z, Zhang S. Label-free electrochemical detection of ATP based on amino-functionalized metal-organic framework. Sci Rep. 2017;7(1):–6500.
Liang G, Man Y, Jin X, Pan L, Liu X, Glökler J. Aptamer-based biosensor for label-free detection of ethanolamine by electrochemical impedance spectroscopy. Anal Chim Acta. 2016;936:222–8.
Istamboulié G, Paniel N, Zara L, Reguillo Granados L, Barthelmebs L, Noguer T. Development of an impedimetric aptasensor for the determination of aflatoxin M1 in milk. Talanta. 2016;146:464–9.
Lao Y-H, Peck K, Chen L-C. Enhancement of aptamer microarray sensitivity through spacer optimization and avidity effect. Anal Chem. 2009;81:1747–54.
Wochner A.. Selektion von Aptameren gegen Antibiotika und deren Einsatz in empfindlichen Assayformaten. Freie Universität Berlin. (2007).
Davis JT. 40 Jahre G-Quartetts: von 5′-GMP zur Molekularbiologie und Supramolekularen Chemie. Angew Chem. 2004;116(6):684–716.
Acknowledgements
The authors gratefully acknowledge the Federal Ministry of Economic Affairs and Energy within the framework of the AiF-ZIM-program for supporting this project under grant nos. ZF4019603MD6 and ZF4086507MD6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Parts of this work were presented at the First European / 10th German BioSensor Symposium, Potsdam, 2017.
Rights and permissions
About this article
Cite this article
Bahner, N., Reich, P., Frense, D. et al. An aptamer-based biosensor for detection of doxorubicin by electrochemical impedance spectroscopy. Anal Bioanal Chem 410, 1453–1462 (2018). https://doi.org/10.1007/s00216-017-0786-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0786-8