Abstract
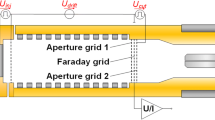
A laser-induced fluorescence (LIF) was used as a complimentary detection system for time-of-flight ion mobility spectrometry (TOF-IMS). A LIF detection system is potentially faster than a conventional electrometer detector and can provide additional (to usual for IMS drift time) analytical information, namely wavelength of fluorescence maxima and fluorescence lifetime. Therefore, better discrimination ability can be expected. Additionally, the combination of IMS and LIF operates at atmospheric pressure. This allows fluorescence measurements of specified ions and ion clusters, which would not survive in a mass spectrometer. An IMS drift cell of open design with both the electrometer and LIF detectors was designed. The feasibility of IMS-LIF was demonstrated on the example of the Xanthene dye Rhodamine 6G (R6G). Electrospray was used as an ionization source. The release and desolvation of R6G ions from the electrospray with following IMS-LIF analysis were demonstrated. The effects of experimental parameters (e.g., ion gate and drift voltages, distance to ESI emitter) are demonstrated and discussed. The obtained results are promising enough to ensure the potential of LIF as a complimentary/alternative detection system for time-of-flight ion mobility spectrometry.






Similar content being viewed by others
References
Johnson AE. Fluorescence approaches for determining protein conformations, interactions and mechanisms at membranes. Traffic. 2005;6(12):1078–92. https://doi.org/10.1111/j.1600-0854.2005.00340.x.
Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312(5771):217–24. https://doi.org/10.1126/science.1124618.
Forbes MW, Jockusch RA. Gas-phase fluorescence excitation and emission spectroscopy of three xanthene dyes (rhodamine 575, rhodamine 590 and rhodamine 6G) in a quadrupole ion trap mass spectrometer. J Am Soc Mass Spectrom. 2011;22(1):93–109. https://doi.org/10.1007/s13361-010-0017-4.
Friedrich J, Fu J, Hendrickson CL, Marshall AG. Time resolved laser-induced fluorescence of electrosprayed ions confined in a linear quadrupole trap. Rev Sci Instrum. 2004;75(11):4511. https://doi.org/10.1063/1.1795111.
Sagoo SK, Jockusch RA. The fluorescence properties of cationic rhodamine B in the gas phase. J Photochem Photobiol A Chem. 2011;220(2–3):173–8. https://doi.org/10.1016/j.jphotochem.2011.04.008.
Chingin K, Chen HW, Gamez G, Zenobi RJ. Exploring fluorescence and fragmentation of ions produced by electrospray ionization in ultrahigh vacuum. Am Soc Mass Spectrom. 2009;20(9):1731–8. https://doi.org/10.1016/j.jasms.2009.05.011.
Frankevich V, Martinez-Lozano Sinues P, Barylyuk K, Zenobi R. Ion mobility spectrometry coupled to laser-induced fluorescence. Anal Chem. 2013;85(1):39–43. https://doi.org/10.1021/ac303137e.
Frankevich VE, Barylyuk KV, Martinez-Lozano Sinues P, Zenobi R. Ion mobility spectrometry coupled to laser-induced fluorescence for probing the electronic structure and conformation of gas-phase ions. J Anal Chem. 2014;69(13):1215–9. https://doi.org/10.1134/S106193481413005X.
SEADM. DMA P5 system, planar differential mobility analyzer. Available at: http://www.seadm.com/wp-content/uploads/2016/09/DMA-P5-SEADM.pdf. Accessed 4 Oct. 2016.
Stephan S, Jakob C, Hippler J, Schmitz OJ. A novel four-dimensional analytical approach for analysis of complex samples. Anal Bioanal Chem. 2016;408(14):3751–9. https://doi.org/10.1007/s00216-016-9460-9.
Kuklya A, Joksimoski S, Kerpen K, Uteschil F, Marks R, Telgheder U. Analysis of gasoline contaminated water samples by means of dopant-assisted atmospheric pressure photoionization differential ion mobility spectrometry. Int. J. Ion Mobil. Spec. 2016;19(2):121–30. https://doi.org/10.1007/s12127-016-0194-3.
Cheng YF, Lu Z, Neue U. Ultrafast liquid chromatography/ultraviolet and liquid chromatography/tandem mass spectrometric analysis. Rapid Commun Mass Spectrom. 2001;15(2):141–51. https://doi.org/10.1002/1097-0231(20010130)15:2<141::AID-RCM201>3.0.CO;2-I.
Eiceman GA, Karpas Z, Hill HH Jr. Ion mobility spectrometry. 3rd ed: CRC Press; 2014.
Borsdorf H, Eiceman GA. Ion mobility spectrometry: principles and applications. Appl Spectrosc Rev. 2006;41(4):323–75. https://doi.org/10.1080/05704920600663469.
Armenta S, Alcala M, Blanco M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal Chim Acta. 2011;703(2):114–23. https://doi.org/10.1016/j.aca.2011.07.021.
Kirk AT, Allers M, Cochems P, Langejuergen J, Zimmermann S. A compact high resolution ion mobility spectrometer for fast trace gas analysis. Analyst. 2013;138(18):5200–7. https://doi.org/10.1039/c3an00231d.
Kirk AT, Zimmermann S. Pushing a compact 15 cm long ultra-high resolution drift tube ion mobility spectrometer with R = 250 to R = 425 using peak deconvolution. Int J Ion Mobil Spec. 2015;18(1):17–22. https://doi.org/10.1007/s12127-015-0166-z.
Guo K, Ni K, Ou G, Zhang X, Yu Q, Qian X, et al. Gaseous phase ion detection method based on laser-induced fluorescence for ion mobility spectrometer. Proceedings of SPIE – The International Society for Optical Engineering. 2015;9621:96210W. https://doi.org/10.1117/12.2193340.
Gormally J, Phillips J. The performance of an ion mobility spectrometer for use with laser ionization. Int J Mass Spectrom Ion Process. 1991;107(3):441–51. https://doi.org/10.1016/0168-1176(91)80040-T.
Bradbury NE, Nielsen RA. Absolute values of the electron mobility in hydrogen. Phys Rev. 1936;49(5):388–93. https://doi.org/10.1103/PhysRev.49.388.
Chingin K, Frankevich V, Balabin RM, Barylyuk K, Chen H, Wang R, et al. Direct access to isolated biomolecules under ambient conditions. Angew Chem Int Ed. 2010;49(13):2358–61. https://doi.org/10.1002/anie.200906213.
Rokushika S, Hatano H, Baim MA, Hill HH Jr. Resolution measurement for ion mobility spectrometry. Anal Chem. 1985;57(9):1902–7. https://doi.org/10.1021/ac00286a023.
Spangler GE, Collins CI. Peak shape analysis and plate theory for plasma chromatography. Anal Chem. 1975;47(3):403–7. https://doi.org/10.1021/ac60353a013.
Acknowledgments
The authors sincerely thank Mr. Jürgen Leistikow and Mr. Heinz-Gerd Müller from the University of Duisburg-Essen for excellent technical assistance. This work was financially supported by the Arbeitsgemeinschaft industrieller Forschungsvereinigungen (AIF), Cologne, (ZIM Project No. KF2210313AK3).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(PDF 893 kb)
Rights and permissions
About this article
Cite this article
Uteschil, F., Kuklya, A., Kerpen, K. et al. Time-of-flight ion mobility spectrometry in combination with laser-induced fluorescence detection system. Anal Bioanal Chem 409, 6279–6286 (2017). https://doi.org/10.1007/s00216-017-0584-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0584-3