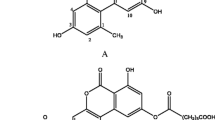
Abstract
A newly developed enzyme immunoassay (EIA) for the detection of the tremorgenic indole-diterpene alkaloid paxilline (PAX) and closely related analogs was used to analyze ergot sclerotia collected from rye and barley fields. The mean EIA standard curve detection limit was 0.47 ± 0.14 ng/mL; relative cross-reactivity of toxin standard solutions was found for 11-hydroxy-paspaline (terpendole E, 1.1%) but not for lolitrem B or ergot alkaloids. Sclerotia from all fields were positive in the PAX-EIA at concentration levels of 620 ± 200 and 160 ± 37 μg/kg in ergot of rye and 130 ± 47 μg/kg in ergot of barley. Confirmatory analyses of sclerotia by liquid chromatography-tandem mass spectrometric detection identified PAX and its analog 13-desoxypaxilline. To the best of our knowledge, this is the first report on the natural occurrence of tremorgenic indole-diterpene alkaloid mycotoxins in ergot sclerotia from rye and barley. Along with details on the analytical methodology developed in this study, particularly PAX-antibody production, the relevance and implications of these findings for food and feed safety are discussed. Presence or absence of elevated levels of tremorgenic mycotoxins, along with the ergot alkaloids, would help in explaining the difference between the two distinct manifestations of historic ergotism, the convulsive and the gangrenous form. Further method development for paxilline and other tremorgenic mycotoxins in cereals used for food and feed is a prerequisite for a comprehensive risk assessment, which seems to be necessary in light of the findings reported here.

Paxilline in ergot of rye






Similar content being viewed by others
References
Steyn PS, Vleggaar R. Tremorgenic mycotoxins. In: Herz W, Grisebach H, Kirby GW, Tamm C, editors. Progress in the chemistry of organic natural products 48. Wien: Springer; 1985. p. 3–80.
Cole RJ, Kirksey JW, Wells JM. A new tremorgenic metabolite of Penicillium paxilli. Can J Microbiol. 1974;20:1159–62.
Springer JP, Clardy J, Wells JM, Cole RJ, Kirksey JW. The structure of paxilline, a tremorgenic metabolite of Penicillium paxilli Bainier. Tetrahedron Lett. 1975;30:2531–4.
Seya H, Nozawa K, Udagawa SI, Nakajima S, Kawai KI. Studies on fungal products. IX. Dethiosecoemestrin, a new metabolite related to emestrin, from Emericella striata. Chem Pharm Bull. 1986;34:2411–6.
Nozawa K, Seyea H, Nakajima S, Udagawa SI, Kawai KI. Studies on fungal products. Part 10. Isolation and structures of novel biocoumarins, desertorins A, B, and C, from Emericella desertorum. J Chem Soc Perkin Trans. I 1987;1735–8.
Nozawa K, Horie Y, Udagawa SI, Kawai KI, Yamazaki M. Isolation of a new tremorgenic indoloditerpene, 1-O′-acetylpaxilline, from Emericella striata and distribution of paxilline in Emericella spp. Chem Pharm Bull. 1989;37:1387–9.
Belofsky GN, Gloer JB. Antiinsectan alkaloids: shearinines A-C and a new paxilline derivative from the ascostromata of Eupenicillium shearii. Tetrahedron. 1995;51:3959–68.
Andersen B, Frisvad JC. Natural occurrence of fungi and fungal metabolites in moldy tomatoes. J Agric Food Chem. 2004;52:7507–13.
Itabashi T, Hosoe T, Wakana D, Fukushima K, Takizawa K, Yaguchi T, et al. A new indoloditerpene derivative, penijanthine A, isolated from Penicillium janthinellum. J Nat Med. 2009;63:96–9.
Uhlig S, Egge-Jacobsen W, Vrålstad T, Miles CO. Indole-diterpenoid profiles of Claviceps paspali and Claviceps purpurea from high-resolution Fourier transform Orbitrap mass spectrometry. Rapid Commun Mass Spectrom. 2014;28:1621–34.
Penn J, Garthwaite I, Christensen MJ, Johnson CM, Towers NR. The importance of paxilline in screening for potentially tremorgenic Acremonium isolates. In: Hume DE, Latch GCM, Easton HS, editors. Proceedings of the second international symposium on Acremonium/grass interactions. AgResearch, Grasslands Research Centre, Private Bag 11008, Palmerston North, New Zealand; 1993. pp. 88–92.
Smith BL, McLeay LM, Embling PP. Effect of the mycotoxins penitrem, paxilline and lolitrem B on the electromyographic activity of skeletal and gastrointestinal smooth muscle of sheep. Res Vet Sci. 1997;62:111–6.
Guerre P. Lolitrem B and indole diterpene alkaloids produced by endophytic fungi of the genus Epichloë and their toxic effects in livestock. Toxins. 2016; 8 Art. 47. doi: 10.3390/toxins8020047.
Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LMH, et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33:5819–28.
Longland CL, Dyer JL, Michelangeli F. The mycotoxin paxilline inhibits the cerebellar inositol 1,4,5-triphosphate receptor. Eur J Pharmacol. 2000;408:219–25.
Bilmen JG, Wootton LL, Michelangeli F. The mechanism of inhibition of the sarco/endoplasmic reticulum Ca2+ ATPase by paxilline. Arch Biochem Biophys. 2002;406:55–64.
Bramlett KS, Houck KA, Borchert KM, Dowless MS, Kulanthaivel P, Zhang Y, et al. A natural product ligand of the oxysterol receptor, liver X receptor. J Pharmacol Exp Ther. 2003;307:291–6.
Sabater-Vilar M, Nijmeijer S, Fink-Gremmels J. Genotoxicity assessment of five tremorgenic mycotoxins (fumitremorgen B, paxilline, penitrem A, verruculogen, and verrucosidin) produced by molds isolated from fermented meats. J Food Prot. 2003;66:2123–9.
Gürbüzel M, Uysal H, Kızılet H. Assessment of genotoxicity of the mycotoxin paxilline using the somatic mutation and recombination test in Drosophila melanogaster. Fresenius Environ Bull. 2010;19:1038–41.
Uhlig S, Botha CJ, Vrålstad T, Rolén E, Miles CO. Indole-diterpenes and ergot alkaloids in Cynodon dactylon (Bermuda grass) infected with Claviceps cynodontis from an outbreak of tremors in cattle. J Agric Food Chem. 2009;57:11112–9.
Negård M, Uhlig S, Kauserud H, Andersen T, Høiland K, Vrålstad T. Links between genetic groups, indole alkaloid profiles and ecology within the grass-parasitic Claviceps purpurea species complex. Toxins. 2015;7:1431–56.
Spatafora JW, Sung GH, Sung JM, Hywel-Jones NL, White JF Jr. Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Mol Ecol. 2007;16:1701–11.
Betina V. Thin-layer chromatography of mycotoxins. J Chromatogr. 1985;334:211–76.
Gallagher RT, Hawkes AD. High-performance liquid chromatography with stop-flow ultraviolet spectral characterization of lolitrem neurotoxins from perennial ryegrass. J Chromatogr. 1985;322:159–67.
Selala MI, Musuku A, Schepens PJC. Isolation and determination of paspalitrem-type tremorgenic mycotoxins using liquid chromatography with diode-array detection. Anal Chim Acta. 1991;244:1–8.
Garthwaite I, Miles CO, Towers NR. Immunological detection of the indole diterpenoid tremorgenic mycotoxins. In: Hume DE, Latch GCM, Easton HS, editors. Proceedings of the second International Symposium on Acremonium/Grass Interactions. AgResearch, Grasslands Research Centre, Private Bag 11008, Palmerston North, New Zealand; 1993. pp. 77–80.
Maragos CM. Development and evaluation of monoclonal antibodies for paxilline. Toxins. 2015:3903–15.
Ackermann Y, Curtui V, Dietrich R, Gross M, Latif H, Märtlbauer E, et al. Widespread occurrence of low levels of alternariol in apple and tomato products, as determined by comparative immunochemical assessment using monoclonal and polyclonal antibodies. J Agric Food Chem. 2011;59:6360–8.
Märtlbauer E, Hack R, Terplan G. Preparation and characterization of polyclonal and monoclonal antibodies against fusarenon-X. Food Agric Immunol. 1989;1:137–46.
Usleber E, Dade M, Schneider E, Dietrich R, Bauer J, Märtlbauer E. Enzyme immunoassay for mycophenolic acid in milk and cheese. J Agric Food Chem. 2008;56:6857–62.
Bauer JI, Gross M, Gottschalk C, Usleber E. Investigations on the occurrence of mycotoxins in beer. Food Control. 2016;63:135–9.
Liesener K, Curtui V, Dietrich R, Märtlbauer E, Usleber E. Mycotoxins in horse feed. Mycotoxin Res. 2010;26:23–30.
Appelt M, Ellner FM. Investigations into the occurrence of alkaloids in ergot and single sclerotia from the 2007 and 2008 harvests. Mycotoxin Res. 2009;25:95–101.
Franzmann C, Schröder J, Mϋnzing K, Wolf K, Lindhauer MG, Humpf HU. Distribution of ergot alkaloids and ricinoleic acid in different milling fractions. Mycotoxin Res. 2011;27:13–21.
Sings H, Singh S. Tremorgenic and nontremorgenic 2,3-fused indole diterpenoids. In: Cordell GA, editor. The alkaloids. San Diego: Academic Press; 2003. p. 51–163.
Goebel-Stengel M, Stengel A, Taché A, Reeve JR Jr. The importance of using the optimal plasticware and glassware in studies involving peptides. Anal Chem. 2011;414:38–46.
Franzmann C, Wächter J, Dittmer N, Humpf HU. Ricinoleic acid as a marker for ergot impurities in rye and rye products. J Agric Food Chem. 2010;58:4223–9.
Malysheva SV, Larionova DA, Di Mavungu JD, De Saeger S. Pattern and distribution of ergot alkaloids in cereals and cereal products from European countries. World Mycotoxin J. 2014;7:217–30.
Commission Regulation (EU) 2015 Commission Regulation (EU) 2015/1940 of 28 October 2015 amending regulation (EC) No 1881/2006 as regards maximum levels of ergot sclerotia in certain unprocessed cereals and the provisions on monitoring and reporting. Off J Eur Union L283:3–6.
Tor-Agbidye J, Blythe LL, Craig AM. Correlation of endophyte toxins (ergovaline and lolitrem B) with clinical disease: fescue foot and perennial ryegrass staggers. Vet Hum Toxicol. 2001;43:140–6.
Barger G. Ergot and ergotism: a monograph based on the Dohme lectures delivered in Johns Hopkins University, Baltimore. London: Gurney and Jackson; 1931. p. 20–84.
Schiff PL. Ergot and its alkaloids. Am J Pharmaceutical Education. 2006; 70:Art. 98.
Eadie MJ. Convulsive ergotism: epidemics of the serotonin syndrome? Lancet Neurol. 2003;2:429–34.
Zhou Y, Lingle CJ. Paxilline inhibits BK channels by an almost exclusively closed-channel block mechanism. J Gen Physiol. 2014;144:415–40.
McMillan LK, Carr RL, Young CA, Astin JW, Lowe RGT, Parker EJ, et al. Molecular analysis of two cytochrome P450 monoxygenase genes required for paxilline biosynthesis in Penicillium paxilli, and effects of paxilline intermediates on mammalian maxi-K ion channels. Mol Gen Genomics. 2003;270:9–23.
Larson BT, Harmon DL, Piper EL, Griffis LM, Bush LP. Alkaloid binding and activation of D2 dopamine receptors in cell culture. J Anim Sci. 1999;77:942–7.
Behrens M, Hüwel S, Galla HJ, Humpf HU. Blood-brain barrier effects of the Fusarium mycotoxins deoxynivalenol, 3 acetyldeoxynivalenol, and moniliformin and their transfer to the brain. PLoS One. 2015; doi:10.1371/journal.pone.0143640.
Kwon OS, Slikker W Jr, Davies DL. Biochemical and morphological effects of fumonisin B1 on primary cultures of rat cerebrum. Neurotoxicol Teratol. 2000;22:565–72.
Belmadani A, Steyn PS, Tramu G, Betbeder AM, Baudrimont I, Creppy EE. Selective toxicity of ochratoxin A in primary cultures from different brain regions. Arch Toxicol. 1999;73:108–14.
Acknowledgments
The authors thank the Brigitte and Wolfram Gedek-Stiftung (Ismaning, Germany) for financial support, Dr. Erwin Röcker for performance of mass spectrometry of PAX and PAX-CMO, and Margit Kessler and Renate Stumpf for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was financially supported by the Brigitte and Wolfram Gedek-Stiftung (Ismaning, Germany).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 636 kb)
Rights and permissions
About this article
Cite this article
Bauer, J.I., Gross, M., Cramer, B. et al. Detection of the tremorgenic mycotoxin paxilline and its desoxy analog in ergot of rye and barley: a new class of mycotoxins added to an old problem. Anal Bioanal Chem 409, 5101–5112 (2017). https://doi.org/10.1007/s00216-017-0455-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0455-y