Abstract
In this article, a facile and sensitive electrochemical method for quantification of Salmonella Pullorum and Salmonella Gallinarum (S. Pullorum and S. Gallinarum) was established by monitoring glucose consumption with a personal glucose meter (PGM). Antibody-functionalized magnetic nanoparticles (IgG-MNPs) were used to capture and enrich S. Pullorum and S. Gallinarum, and IgG-MNPs-S. Pullorum and IgG-MNPs-S. Gallinarum complexes were magnetically separated from a sample using a permanent magnet. The trace tag was prepared by loading polyclonal antibodies and high-content glucose oxidase on amino-functionalized silica nanoparticles (IgG-SiNPs-GOx). With a sandwich-type immunoassay format, IgG-SiNPs-GOx were added into the above mixture solution and conjugated to the complexes, forming sandwich composites IgG-MNPs/S. Pullorum and S. Gallinarum/IgG-SiNPs-GOx. The above sandwich composites were dispersed in glucose solution. Before and after the hydrolysis of glucose, the concentration of glucose was measured using PGM. Under optimal conditions, a linear relationship between the decrease of glucose concentration and the logarithm of S. Pullorum and S. Gallinarum concentration was obtained in the concentration range from 1.27 × 102 to 1.27 × 105 CFU mL−1, with a detection limit of 7.2 × 101 CFU mL−1 (S/N = 3). This study provides a portable, low-cost, and quantitative analytical method for bacteria detection; thus, it has a great potential in the prevention of disease caused by S. Pullorum and S. Gallinarum in poultry.

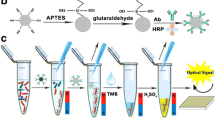
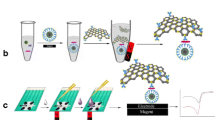
A schematic illustration of the fabrication process of IgG-SiNPs-GOD nanomaterials (A) and IgG-MNPs (B) and experimental procedure of detection of S. Pullorum and S. Gallinarum using GOD-functionalized silica nanospheres as trace tags based on PGM (C).










Similar content being viewed by others
References
Lee K-M, Runyon M, Herrman TJ, Phillips R, Hsieh J. Review of Salmonella detection and identification methods: aspects of rapid emergency response and food safety. Food Control. 2015;47:264–76.
Hu C, Dou W, Zhao G. Enzyme immunosensor based on gold nanoparticles electroposition and streptavidin-biotin system for detection of S. pullorum & S. gallinarum. Electrochim Acta. 2014;117:239–45.
Wan Y, Qi P, Zhang D, Wu J, Wang Y. Manganese oxide nanowire-mediated enzyme-linked immunosorbent assay. Biosens Bioelectron. 2012;33(1):69–74.
Soria MA, Soria MC, Bueno DJ. A comparative study of culture methods and polymerase chain reaction for Salmonella detection in egg content. Poult Sci. 2012;91(10):2668–76.
Ozalp VC, Bayramoglu G, Erdem Z, Arica MY. Pathogen detection in complex samples by quartz crystal microbalance sensor coupled to aptamer functionalized core-shell type magnetic separation. Anal Chim Acta. 2015;853:533–40.
Vaisocherova-Lisalova H, Visova I, Ermini ML, Springer T, Song XC, Mrazek J, Lamacova J, Scott Lynn Jr N, Sedivak P, Homola J. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens Bioelectron. 2016;80:84–90.
Mouffouk F, da Costa AM, Martins J, Zourob M, Abu-Salah KM, Alrokayan SA. Development of a highly sensitive bacteria detection assay using fluorescent pH-responsive polymeric micelles. Biosens Bioelectron. 2011;26(8):3517–23.
Zhou H, Yang D, Mircescu NE, Ivleva NP, Schwarzmeier K, Wieser A, Schubert S, Niessner R, Haisch C. Surface-enhanced Raman scattering detection of bacteria on microarrays at single cell levels using silver nanoparticles. Microchim Acta. 2015;182(13–14):2259–66.
Yang W, Lu X, Wang Y, Sun S, Liu C, Li Z. Portable and sensitive detection of protein kinase activity by using commercial personal glucose meter. Sens Actuators B Chem. 2015;210:508–12.
Ming J, Fan W, Jiang T-F, Wang Y-H, Lv Z-H. Portable and sensitive detection of copper(II) ion based on personal glucose meters and a ligation DNAzyme releasing strategy. Sens Actuators B Chem. 2017;240:1091–8.
Xiang Y, Lu Y. Using commercially available personal glucose meters for portable quantification of DNA. Anal Chem. 2012;84(4):1975–80.
Xiang Y, Lu Y. Portable and quantitative detection of protein biomarkers and small molecular toxins using antibodies and ubiquitous personal glucose meters. Anal Chem. 2012;84(9):4174–8.
Ma X, Chen Z, Zhou J, Weng W, Zheng O, Lin Z, Guo L, Qiu B, Chen G. Aptamer-based portable biosensor for platelet-derived growth factor-BB (PDGF-BB) with personal glucose meter readout. Biosens Bioelectron. 2014;55:412–6.
Joo J, Kwon D, Shin HH, Park K-H, Cha HJ, Jeon S. A facile and sensitive method for detecting pathogenic bacteria using personal glucose meters. Sens Actuators B Chem. 2013;188:1250–4.
Ávila EFD, Escamilla-Gómez V, Campuzano S, Pedrero M, Salvador JP, Marco MP, Pingarrón JM. Ultrasensitive amperometric magnetoimmunosensor for human C-reactive protein quantification in serum. Sens Actuators B Chem. 2013;188(11):212–20.
Liu X, Shuai H-L, Liu Y-J, Huang K-J. An electrochemical biosensor for DNA detection based on tungsten disulfide/multi-walled carbon nanotube composites and hybridization chain reaction amplification. Sens Actuators B Chem. 2016;235:603–13.
Datta S, Christena LR, Rajaram YRS. Enzyme immobilization: an overview on techniques and support materials. 3 Biotech. 2013;3(1):1–9.
Kim S, Ko J, Lim HB. Application of magnetic and core-shell nanoparticles to determine enrofloxacin and its metabolite using laser induced fluorescence microscope. Anal Chim Acta. 2013;771:37–41.
Ke R, Yang W, Xia X, Xu Y, Li Q. Tandem conjugation of enzyme and antibody on silica nanoparticle for enzyme immunoassay. Anal Biochem. 2010;406(1):8–13.
Chen L, Zhang Z, Zhang X, Fu A, Xue P, Yan R. A novel chemiluminescence immunoassay of staphylococcal enterotoxin B using HRP-functionalised mesoporous silica nanoparticle as label. Food Chem. 2012;135(1):208–12.
Tang D, Su B, Tang J, Ren J, Chen G. Nanoparticle-based sandwich electrochemical immunoassay for carbohydrate antigen 125 with signal enhancement using enzyme-coated nanometer-sized enzyme-doped silica beads. Anal Chem. 2010;82(4):1527–34.
Wu Y, Chen C, Liu S. Enzyme-functionalized silica nanoparticles as sensitive labels in biosensing. Anal Chem. 2009;81(4):1600–7.
Lu Z, Wang G, Zhuang J, Yang W. Effects of the concentration of tetramethylammonium hydroxide peptizer on the synthesis of Fe3O4/SiO2 core/shell nanoparticles. Colloids Surf A Physicochem Eng Asp. 2006;278(1–3):140–3.
Bagwe RP, Yang C, Hilliard LR, Tan W. Optimization of dye-doped silica nanoparticles prepared using a reverse microemulsion method. Langmuir. 2004;20(19):8336–42.
Tapec R, Zhao XJ, Tan W. Development of organic dye-doped silica nanoparticles for bioanalysis and biosensors. J Nanosci Nanotechnol. 2002;2(2):405–9.
Sun Q, Zhao G, Dou W. An optical and rapid sandwich immunoassay method for detection of Salmonella pullorum and Salmonella gallinarum based on immune blue silica nanoparticles and magnetic nanoparticles. Sens Actuators B Chem. 2016;226:69–75.
Shukla S, Leem H, Kim M. Development of a liposome-based immunochromatographic strip assay for the detection of Salmonella. Anal Bioanal Chem. 2011;401(8):2581–90.
Shim W-B, Song J-E, Mun H, Chung D-H, Kim M-G. Rapid colorimetric detection of Salmonella typhimurium using a selective filtration technique combined with antibody–magnetic nanoparticle nanocomposites. Anal Bioanal Chem. 2014;406(3):859–66.
Wen C-Y, Hu J, Zhang Z-L, Tian Z-Q, Ou G-P, Liao Y-L, Li Y, Xie M, Sun Z-Y, Pang D-W. One-step sensitive detection of Salmonella typhimurium by coupling magnetic capture and fluorescence identification with functional nanospheres. Anal Chem. 2013;85(2):1223–30.
Fei J, Dou W, Zhao G. A sandwich electrochemical immunosensor for Salmonella pullorum and Salmonella gallinarum based on a screen-printed carbon electrode modified with an ionic liquid and electrodeposited gold nanoparticles. Microchim Acta. 2015;182(13–14):2267–75.
Soria MC, Soria MA, Bueno DJ, Terzolo HR. Comparison of 3 culture methods and PCR assays for Salmonella gallinarum and Salmonella pullorum detection in poultry feed. Poult Sci. 2013;92(6):1505–15.
Ho J-aA, Zeng S-C, Tseng W-H, Lin Y-J, C-h C. Liposome-based immunostrip for the rapid detection of Salmonella. Anal Bioanal Chem. 2008;391(2):479–85.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of Zhejiang Province (LY17C2000003), the Food Science and Engineering the most important discipline of Zhejiang Province (JYTSP20141062), the Zhejiang Public Innovation Platform Analysis and Testing Project (2015C37023), the Talent Training Provincial Superior Paper Funded Project (1110JY1412001P), the Technological Innovation Project of Zhejiang Gongshang University (CX201610024, CX201610019), the Graduate Research Innovation Fund Project of Zhejiang Gongshang University, and the Plans of College Students in Zhejiang Province and Technology Innovation Activities (Acrobatic Tender Grass Talent Programme) Project (1110JQ4212048G, 1110KZN0213112G).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 90.7 kb)
Rights and permissions
About this article
Cite this article
Luo, Y., Dou, W. & Zhao, G. Rapid electrochemical quantification of Salmonella Pullorum and Salmonella Gallinarum based on glucose oxidase and antibody-modified silica nanoparticles. Anal Bioanal Chem 409, 4139–4147 (2017). https://doi.org/10.1007/s00216-017-0361-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0361-3