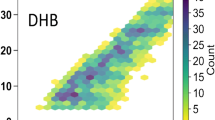
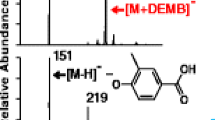
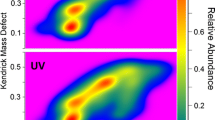
Abstract
Chemical degradation is an efficient method to obtain bio-oils and other compounds from lignin. Lignin bio-oils are potential substitutes for the phenol component of phenol formaldehyde (PF) resins. Here, we developed an analytical method based on high resolution mass spectrometry that provided structural information for the synthesized lignin-derived resins and supported the prediction of their properties. Different model resins based on typical lignin degradation products were analyzed by electrospray ionization in negative ionization mode. Utilizing enhanced mass defect filter techniques provided detailed structural information of the lignin-based model resins and readily complemented the analytical data from differential scanning calorimetry and thermogravimetric analysis. Relative reactivity and chemical diversity of the phenol substitutes were significant determinants of the outcome of the PF resin synthesis and thus controlled the areas of application of the resulting polymers.

ᅟ




Similar content being viewed by others
References
Megson NJL. Aldehyde phenolic condensations from a chemical standpoint. Trans Faraday Soc. 1935;336–345.
Knop A, Pilato LA. Phenolic resins: chemistry, applications and performance. Berlin Heidelberg: Springer-Verlag; 2013.
Hultzsch K. Chemie der Phenolharze. Berlin Heidelberg: Springer-Verlag; 1950.
Gardziella A, Pilato L, Knop A. Phenolic resins : chemistry, applications, standardization, safety, and ecology. Berlin Heidelberg: Springer-Verlag; 2013.
Pilato LA. Phenolic resins: a century of progress. Berlin Heidelberg: Springer-Verlag; 2010.
BP. Statistical review of world energy. 2016;1–48.
Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, et al. Lignin valorization: improving lignin processing in the biorefinery. Science. 2014;344:1246843.
Pradhan SK, Chakraborty I, Kar BB. Chemically modified lignin—a potential resource material for composites with better stability. Int J Sci Environ Technol. 2015;4:183–9.
Wang H, Tucker M, Ji Y. Recent development in chemical depolymerization of lignin: a review. J Appl Chem. 2013;2013:1–9.
Pandey MP, Kim CS. Lignin depolymerization and conversion: a review of thermochemical methods. Chem Eng Technol. 2011;34:29–41.
de Wild PJ, Huijgen WJJ, Gosselink RJA. Perspective: lignin pyrolysis for profitable lignocellulosic biorefineries. Biofuels, Bioprod Biorefining. 2012;6:246–56.
Mudraboyina BP, Fu D, Jessop PG. Supercritical fluid rectification of lignin microwave-pyrolysis oil. Green Chem. 2015;17:169–72.
Jegers HE, Klein MT. Primary and secondary lignin pyrolysis reaction pathways. Ind Eng Chem Process Des Dev. 1985;24:173–83.
Forchheim D, Hornung U, Kempe P, Kruse A, Steinbach D. Influence of RANEY nickel on the formation of intermediates in the degradation of lignin. Int J Chem Eng. 2012;2012:589749.
Stewart D. Lignin as a base material for materials applications: chemistry, application and economics. Ind Crops Prod. 2008;27:202–7.
Kleinert M, Barth T. Phenols from lignin. Chem Eng Technol. 2008;31:736–45.
Pineda A, Lee AF. Heterogeneously catalyzed lignin depolymerization. Appl Petrochem Res. 2016;6:1–14.
Deepa AK, Dhepe PL. Lignin depolymerization into aromatic monomers over solid acid catalysts. ACS Catal. 2015;5:365–79.
Sprung MM. Reactivity of phenols toward paraformaldehyde. J Am Chem Soc. 1941;63:334–43.
Mitsunaga T, Conner AH, Hill CG. Reaction of formaldehyde with phenols: a computational study. Wood Adhes. 2000;147–154.
Cheng S, Yuan Z, Leitch M, Anderson M, Xu CC. Highly efficient de-polymerization of organosolv lignin using a catalytic hydrothermal process and production of phenolic resins/adhesives with the depolymerized lignin as a substitute for phenol at a high substitution ratio. Ind Crops Prod. 2013;44:315–22.
Feng S, Yuan Z, Leitch M, Shui H, Xu CC. Effects of bark extraction before liquefaction and liquid oil fractionation after liquefaction on bark-based phenol formaldehyde resoles. Ind Crops Prod. 2016;84:330–6.
Moubarik A, Grimi N, Boussetta N, Pizzi A. Isolation and characterization of lignin from Moroccan sugar cane bagasse: production of lignin-phenol-formaldehyde wood adhesive. Ind Crops Prod. 2013;45:296–302.
Turunen M, Alvila L, Pakkanen TT, Rainio J. Modification of phenol-formaldehyde resol resins by lignin, starch, and urea. J Appl Polym Sci. 2003;88:582–8.
Wang M, Leitch M, Xu C. Synthesis of phenol-formaldehyde resol resins using organosolv pine lignins. Eur Polym J. 2009;45:3380–8.
Yuan FY, Zhang HB, Li X, Ma HL, Li XZ, Yu ZZ. In situ chemical reduction and functionalization of graphene oxide for electrically conductive phenol formaldehyde composites. Carbon. 2014;68:653–61.
Liu C, Li K, Li H, Zhang S, Zhang Y. The effect of zirconium incorporation on the thermal stability and carbonized product of phenol-formaldehyde resin. Polym Degrad Stab. 2014;102:180–5.
Yang S, Zhang Y, Yuan T-Q, Sun R-C. Lignin-phenol-formaldehyde resin adhesives prepared with biorefinery technical lignins. J Appl Polym Sci. 2015;132:42493.
Tachon N, Benjelloun-Mlayah B, Delmas M. Organosolv wheat straw lignin as a phenol substitute for green phenolic resins. BioResources. 2016;11:5797–815.
Hoong YB, Pizzi A, Chuah LA, Harun J. Phenol-urea-formaldehyde resin co-polymer synthesis and its influence on elaeis palm trunk plywood mechanical performance evaluated by 13C NMR and MALDI-TOF mass spectrometry. Int J Adhes Adhes. 2015;63:117–23.
Wang J, Jiang H, Jiang N. Study on the pyrolysis of phenol-formaldehyde (PF) resin and modified PF resin. Thermochim Acta. 2009;496:136–42.
Sobera M, Hetper J. Pyrolysis-gas chromatography-mass spectrometry of cured phenolic resins. J Chromatogr A. 2003;993:131–5.
Strzemiecka B, Voelkel A, Zieba-Palus J, Lachowicz T. Assessment of the chemical changes during storage of phenol-formaldehyde resins pyrolysis gas chromatography mass spectrometry, inverse gas chromatography and Fourier transform infra red methods. J Chromatogr A. 2014;1359:255–61.
Santos RM B, Martinho Simões JA. Energetics of the O-H bond in phenol and substituted phenols: a critical evaluation of literature data. J Phys Chem Ref Data. 1998;27:707–39.
Dier TKF, Egele K, Fossog V, Hempelmann R, Volmer DA. Enhanced mass defect filtering to simplify and classify complex mixtures of lignin degradation products. Anal Chem. 2016;88:1328–35.
Qi Y, Hempelmann R, Volmer DA. Two-dimensional mass defect matrix plots for mapping genealogical links in mixtures of lignin depolymerisation products. Anal Bioanal Chem. 2016;408:4835–43.
Qi Y, Hempelmann R, Volmer DA. Shedding light on the structures of lignin compounds: photo-oxidation under artificial UV light and characterization by high resolution mass spectrometry. Anal Bioanal Chem. 2016;408:8203–10.
Kendrick E. A mass scale based on CH, = 14. 0000 for high resolution mass spectrometry of organic compounds. Anal Biochem. 1963;35:2146–54.
Determination of free-formaldehyde content by hydroxylamine hydrochloride method (ISO 9397:1995). 1997. https://www.beuth.de/de/norm/din-en-iso-9397/2973638.
Hathway DE, Seakins JWT. Autoxidation of catechin. Nature. 1955;176:218.
Pekala RW. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J Mater Sci. 1989;24:3221–7.
Li P, Coleman DW, Spaulding KM, McClennen WH, Stafford PR, Fife DJ. Fractionation and characterization of phenolic resins by high-performance liquid chromatography and gel-permeation chromatography combined with ultraviolet, refractive index, mass spectrometry and light-scattering detection. J Chromatogr A. 2001;914:147–59.
Christiansen AW, Gollob L. Differential scanning calorimetry of phenol-formaldehyde resols. J Appl Polym Sci. 1985;30:2279–89.
Khan MA, Ashraf SM, Malhotra VP. Eucalyptus bark lignin substituted phenol formaldehyde adhesives: a study on optimization of reaction parameters and characterization. J Appl Polym Sci. 2004;92:3514–23.
Acknowledgements
The authors acknowledge the Alfried Krupp von Bohlen und Halbach-Stiftung, the German Research Foundation (DFG VO 1355/4-1 and FTICR-MS Facility, DFG INST 256/356-1) and “Niedersächsisches Ministerium für Wissenschaft und Kultur” for financial support. The authors thank Allnex S.à.r.l. (Luxembourg) for the GPC experiments.
Author contributions
The manuscript was written through contributions of all authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial or non-financial interests.
Additional information
Tobias K. F. Dier and Marco Fleckenstein contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dier, T.K.F., Fleckenstein, M., Militz, H. et al. Exploring the potential of high resolution mass spectrometry for the investigation of lignin-derived phenol substitutes in phenolic resin syntheses. Anal Bioanal Chem 409, 3441–3451 (2017). https://doi.org/10.1007/s00216-017-0282-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0282-1