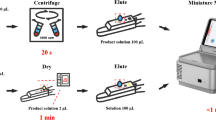
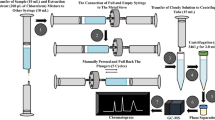
Abstract
Matrix effects have been a major concern when developing LC−MS/MS methods for quantitative bioanalysis of cycloserine. Sample handling procedures including solid phase extraction or derivatization have been reported previously by researchers to overcome matrix effects of cycloserine. In the present study, the possibility of reducing matrix effects of cycloserine using protein precipitation coupled with dilution techniques was investigated. Plasma samples were pretreated by protein precipitation with methanol followed by a 40-fold dilution with methanol–water (50:50, v/v). The analyte and the internal standard (mildronate) were chromatographed on a Shim-pack XR-ODS (100 mm × 2.0 mm, 2.2 μm) column using methanol–0.01% formic acid (70:30, v/v) as mobile phase and detected by multiple reaction monitoring mode via positive electrospray ionization. The total run time was only 2 min per sample. The suppression of cycloserine response was reduced with the matrix effects ranging between 80.5 and 87.9%. A lower limit of quantification (LLOQ) of 0.300 μg/mL was achieved using only 10 μL of plasma. The intra- and inter-day precisions were less than 4.8% and the accuracy ranged from −2.6 to 6.6%. The method was successfully applied to a pharmacokinetic study of cycloserine in 30 healthy Chinese male subjects after oral administration of a single dose of cycloserine at 250, 500 and 750 mg under fasting conditions. The newly developed method is simpler, faster, cost-effective, and more robust than previously reported LC-MS/MS methods.





Similar content being viewed by others
References
Zygmunt WA. Antagonism of D-cycloserine inhibition of mycobacterial growth by D-alanine. J Bacteriol. 1963;85:1217–20.
Johnson R, Streicher EM, Louw GE, Warren RM, van Helden PD, Victor TC. Drug resistance in mycobacterium tuberculosis. Curr Issues Mol Biol. 2006;8:97–112.
Abubakar I, Moore J, Drobniewski F, Kruijshaar M, Brown T, Yates M, et al. Extensively drug-resistant tuberculosis in the UK: 1995 to 2007. Thorax. 2009;64(6):512–5.
Patel DS, Sharma N, Patel MC, Patel BN, Shrivastav PS, Sanyal M. Development and validation of a selective and sensitive LC−MS/MS method for determination of cycloserine in human plasma: application to bioequivalence study. J Chromatogr B. 2011;879(23):2265–73.
Yaroshenko DV, Grigoriev AV, Sidorova AA. Development and validation of a LC−MS/MS method for D-cycloserine determination in human plasma for bioequivalence study. Anal Bioanal Chem. 2014;406(3):923–7.
Polagani SR, Pilli NR, Maddela R, Gajula R, Gandu V. A rapid and sensitive liquid chromatography-tandem mass spectrometric assay for cycloserine in 50μL of human plasma: its pharmacokinetic application. J Pharm Biomed Anal. 2013;76:21–7.
Stepanova ES, Ovcharov MV, Barsegyan SS, Chistyakov VV. Determination of cycloserine in blood plasma by HPLC/MS: application to bioequivalence studies. Pharm Chem J. 2016;50(3):195–9.
Lee D, Garrett TJ, Goldberger BA, Bazydlo LA. Quantitation of 25-hydroxyvitamin D2 and D3 in serum and plasma by LCMS/MS. Bioanalysis. 2015;7(2):167–78.
Ding J, Jin G, Jin G, Shen A, Guo Z, Yu B, et al. Determination of underivatized glyphosate residues in plant-derived food with low matrix effect by solid phase extraction-liquid chromatography-tandem mass spectrometry. Food Anal Method. 2016;9:2856–63.
Isaguirre AC, Olsina RA, Martinez LD, Lapierre AV, Cerutti S. Development of solid phase extraction strategies to minimize the effect of human urine matrix effect on the response of carnitine by UPLC–MS/MS. Microchem J. 2016;129:362–7.
Havlíková L, Vlčková H, Solich P, Nováková L. HILIC UHPLC-MS/MS for fast and sensitive bioanalysis: accounting for matrix effects in method development. Bioanalysis. 2013;5(19):2345–57.
Wu Y, Mao Z, Liu Y, Wang X, Di X. Simultaneous determination of febuxostat and its three active metabolites in human plasma by liquid chromatography-tandem mass spectrometry and its application to a pharmacokinetic study in Chinese healthy volunteers. J Pharm Biomed Anal. 2015;114:216–21.
Forteschi M, Zinellu A, Assaretti S, Mangoni AA, Pintus G, Carru C, et al. An isotope dilution capillary electrophoresis/tandem mass spectrometry (CE-MS/MS) method for the simultaneous measurement of choline, betaine, and dimethylglycine concentrations in human plasma. Anal Bioanal Chem. 2016;408(26):7505–12.
Meisser Redeuil K, Longet K, Bénet S, Munari C, Campos-Giménez E. Simultaneous quantification of 21 water soluble vitamin circulating forms in human plasma by liquid chromatography-mass spectrometry. J Chromatogr A. 2015;1422:89–98.
Food and drug Administration (2013) Guidance for industry: bioanalytical method validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf. Accessed 16 Feb 2015.
Ferrer C, Lozano A, Agüera A, Girón AJ, Fernández-Alba AR. Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables. J Chromatogr A. 2011;1218(42):7634–9.
Ekdahl A, Johansson MC, Ahnoff M. Tracing and separating plasma components causing matrix effects in hydrophilic interaction chromatography-electrospray ionization mass spectrometry. J Chromatogr B. 2013;923–924:83–91.
Fraschetti C, Filippi A, Mannina L, Sobolev AP, Speranza M. Role of the solvent on the stability of cycloserine under ESI-MS conditions. J Mass Spectrom. 2014;49(7):608–12.
Acknowledgements
The authors are thankful to Ms. Na Zhao and Ms. Yang Liu of Yiling Medical Technology Co., Ltd. for their technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study has been approved by the People’s Hospital of Liaoning Province Ethics Committee and has been performed in accordance with the ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was provided by all individuals involved with the study.
Rights and permissions
About this article
Cite this article
Mao, Z., Wang, X., Li, B. et al. A simplified LC−MS/MS method for rapid determination of cycloserine in small-volume human plasma using protein precipitation coupled with dilution techniques to overcome matrix effects and its application to a pharmacokinetic study. Anal Bioanal Chem 409, 3025–3032 (2017). https://doi.org/10.1007/s00216-017-0249-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0249-2