Abstract
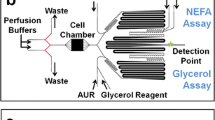
Microfluidics is an enabling technology for both cell biology and chemical analysis. We combine these attributes with a microfluidic device for on-line solid-phase extraction (SPE) and mass spectrometry (MS) analysis of secreted metabolites from living cells in culture on the chip. The device was constructed with polydimethylsiloxane (PDMS) and contains a reversibly sealed chamber for perfusing cells. A multilayer design allowed a series of valves to control an on-chip 7.5 μL injection loop downstream of the cell chamber with operation similar to a six-port valve. The valve collects sample and then diverts it to a packed SPE bed that was connected in-line to treat samples prior to MS analysis. The valve allows samples to be collected and injected onto the SPE bed while preventing exposure of cells to added back pressure from the SPE bed and organic solvents needed to elute collected chemicals. Here, cultured murine 3T3-L1 adipocytes were loaded into the cell chamber and non-esterified fatty acids (NEFAs) that were secreted by the cells were monitored by SPE-MS at 30 min intervals. The limit of detection for a palmitoleic acid standard was 1.4 μM. Due to the multiplexed detection capabilities of MS, a variety of NEFAs were detected. Upon stimulation with isoproterenol and forskolin, secretion of select NEFAs was elevated an average of 1.5-fold compared to basal levels. Despite the 30-min delay between sample injections, this device is a step towards a miniaturized system that allows automated monitoring and identification of a variety of molecules in the extracellular environment.






Similar content being viewed by others
References
Nge PN, Rogers CI, Woolley AT. Advances in microfluidic materials, functions, integration, and applications. Chem Rev. 2013;113:2550–83.
Roman GT, Kennedy RT. Fully integrated microfluidic separations systems for biochemical analysis. J Chromatogr A. 2007;1168:170–88.
Hung PJ, Lee PJ, Sabounchi P, Aghdam N, Lin R, Lee LP. A novel high aspect ratio microfluidic design to provide a stable and uniform microenvironment for cell growth in a high throughput mammalian cell culture array. Lab Chip. 2005;5:44–8.
Kim L, Toh YC, Voldman J, Yu H. A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip. 2007;7:681–94.
El-Ali J, Sorger PK, Jensen KF. Cells on chips. Nature. 2006;442:403–11.
Vyawahare S, Griffiths AD, Merten CA. Miniaturization and parallelization of biological and chemical assays in microfluidic devices. Chem Biol. 2010;17:1052–65.
Salieb-Beugelaar GB, Simone G, Arora A, Philippi A, Manz A. Latest developments in microfluidic cell biology and analysis systems. Anal Chem. 2010;82:4848–64.
Primiceri E, Chiriaco MS, Rinaldi R, Maruccio G. Cell chips as new tools for cell biology—results, perspectives and opportunities. Lab Chip. 2013;13:3789–802.
Culbertson CT, Mickleburgh TG, Stewart-James SA, Sellens KA, Pressnall M. Micro total analysis systems: fundamental advances and biological applications. Anal Chem. 2014;86:95–118.
Walker GM, Zeringue HC, Beebe DJ. Microenvironment design considerations for cellular scale studies. Lab Chip. 2004;4:91–7.
Dhumpa R, Roper MG. Temporal gradients in microfluidic systems to probe cellular dynamics: a review. Anal Chim Acta. 2012;743:9–18.
Kovarik ML, Gach PC, Ornoff DM, Wang Y, Balowski J, Farrag L, et al. Micro total analysis systems for cell biology and biochemical assays. Anal Chem. 2012;84:516–40.
Sankar KS, Green BJ, Crocker AR, Verity JE, Altamentova SM, Rocheleau JV. Culturing pancreatic islets in microfluidic flow enhances morphology of the associated endothelial cells. PLoS ONE. 2011;6.
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239.
Hung PJ, Lee PJ, Sabounchi P, Lin R, Lee LP. Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol Bioeng. 2005;89:1–8.
Huh D, Fujioka H, Tung Y-C, Futai N, Paine R, Grotberg JB, et al. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci U S A. 2007;104:18886–91.
Godwin LA, Pilkerton ME, Deal KS, Wanders D, Judd RL, Easley CJ. Passively operated microfluidic device for stimulation and secretion sampling of single pancreatic islets. Anal Chem. 2011;83:7166–72.
Croushore CA, Supharoek S-A, Lee CY, Jakmunee J, Sweedler JV. Microfluidic device for the selective chemical stimulation of neurons and characterization of peptide release with mass spectrometry. Anal Chem. 2012;84:9446–52.
Kane BJ, Zinner MJ, Yarmush ML, Toner M. Liver-specific functional studies in a microfluidic array of primary mammalian hepatocytes. Anal Chem. 2006;78:4291–8.
Godwin LA, Brooks JC, Hoepfner LD, Wanders D, Judd RL, Easley CJ. A microfluidic interface for the culture and sampling of adiponectin from primary adipocytes. Analyst. 2015;140:1019–25.
Ong TH, Tillmaand EG, Makurath M, Rubakhin SS, Sweedler JV. Mass spectrometry-based characterization of endogenous peptides and metabolites in small volume samples. BBA-Proteins Proteomics. 1854;2015:732–40.
Goto M, Sato K, Murakami A, Tokeshi M, Kitamori T. Development of a microchip-based bioassay system using cultured cells. Anal Chem. 2005;77:2125–31.
Shackman JG, Dahlgren GM, Peters JL, Kennedy RT. Perfusion and chemical monitoring of living cells on a microfluidic chip. Lab Chip. 2005;5:56–63.
Shackman JG, Reid KR, Dugan CE, Kennedy RT. Dynamic monitoring of glucagon secretion from living cells on a microfluidic chip. Anal Bioanal Chem. 2012;402:2797–803.
Dishinger JF, Reid KR, Kennedy RT. Quantitative monitoring of insulin secretion from single islets of Langerhans in parallel on a microfluidic chip. Anal Chem. 2009;81:3119–27.
Clark AM, Sousa KM, Chisolm CN, MacDougald OA, Kennedy RT. Reversibly sealed multilayer microfluidic device for integrated cell perfusion and on-line chemical analysis of cultured adipocyte secretions. Anal Bioanal Chem. 2010;397:2939–47.
Clark AM, Sousa KM, Jennings C, MacDougald OA, Kennedy RT. Continuous-flow enzyme assay on a microfluidic chip for monitoring glycerol secretion from cultured adipocytes. Anal Chem. 2009;81:2350–6.
Dugan CE, Cawthorn WP, MacDougald OA, Kennedy RT. Multiplexed microfluidic enzyme assays for simultaneous detection of lipolysis products from adipocytes. Anal Bioanal Chem. 2014;406:4851–9.
Bowen AL, Martin RS. Integration of on-chip peristaltic pumps and injection valves with microchip electrophoresis and electrochemical detection. Electrophoresis. 2010;31:2534–40.
Price AK, Fischer DJ, Martin RS, Spence DM. Deformation-induced release of ATP from erythrocytes in a poly(dimethylsiloxane)-based microchip with channels that mimic resistance vessels. Anal Chem. 2004;76:4849–55.
Nunemaker CS, Dishinger JF, Dula SB, Wu R, Merrins MJ, Reid KR, et al. Glucose metabolism, islet architecture, and genetic homogeneity in imprinting of [Ca2+]i and insulin rhythms in mouse islets. PLoS ONE. 2009;4, e8428.
Wang X, Yi L, Mukhitov N, Schrell AM, Dhumpa R, Roper MG. Microfluidics-to-mass spectrometry: a review of coupling methods and applications. J Chromatogr A. 2015;1382:98–116.
Nge PN, Pagaduan JV, Yu M, Woolley AT. Microfluidic chips with reversed-phase monoliths for solid phase extraction and on-chip labeling. J Chromatogr A. 2012;1261:129–35.
Mellors JS, Gorbounov V, Ramsey RS, Ramsey JM. Fully integrated glass microfluidic device for performing high-efficiency capillary electrophoresis and electrospray ionization mass spectrometry. Anal Chem. 2008;80:6881–7.
Lazar IM, Trisiripisal P, Sarvaiya HA. Microfluidic liquid chromatography system for proteomic applications and biomarker screening. Anal Chem. 2006;78:5513–24.
Liuni P, Rob T, Wilson DJ. A microfluidic reactor for rapid, low-pressure proteolysis with on-chip electrospray ionization. Rapid Commun Mass Spec. 2010;24:315–20.
Ohla S, Belder D. Chip-based separation devices coupled to mass spectrometry. Curr Opin Chem Biol. 2012;16:453–9.
Li X, Hu H, Zhao S, Liu Y-M. Microfluidic platform with in-chip electrophoresis coupled to mass spectrometry for monitoring neurochemical release from nerve cells. Anal Chem. 2016;88:5338–44.
Enders JR, Marasco CC, Wikswo JP, McLean JA. A dual-column solid phase extraction strategy for online collection and preparation of continuously flowing effluent streams for mass spectrometry. Anal Chem. 2012;84:8467–74.
Gasilova N, Qiao L, Momotenko D, Pourhaghighi MR, Girault HH. Microchip emitter for solid-phase extraction–gradient elution–mass spectrometry. Anal Chem. 2013;85:6254–63.
Kumar S, Sahore V, Rogers CI, Woolley AT. Development of an integrated microfluidic solid-phase extraction and electrophoresis device. Analyst. 2016;141:1660–8.
Gao D, Wei H, Guo G-S, Lin J-M. Microfluidic cell culture and metabolism detection with electrospray ionization quadrupole time-of-flight mass spectrometer. Anal Chem. 2010;82:5679–85.
Wei H, Li H, Gao D, Lin J-M. Multi-channel microfluidic devices combined with electrospray ionization quadrupole time-of-flight mass spectrometry applied to the monitoring of glutamate release from neuronal cells. Analyst. 2010;135:2043–50.
Luni C, Giulitti S, Serena E, Ferrari L, Zambon A, Gagliano O, et al. High-efficiency cellular reprogramming with microfluidics. Nat Methods. 2016;13:446–52.
Patabadige DEW, Mickleburgh T, Ferris L, Brummer G, Culbertson AH, Culbertson CT. High-throughput microfluidic device for single cell analysis using multiple integrated soft lithographic pumps. Electrophoresis. 2016;37:1337–44.
Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000;288:113–6.
Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endoc Metab. 2005;19:471–82.
Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–97.
Cellar NA, Burns ST, Meiners J-C, Chen H, Kennedy RT. Microfluidic chip for low-flow push-pull perfusion sampling in vivo with on-line analysis of amino acids. Anal Chem. 2005;77:7067–73.
Ceriotti L, de Rooij NF, Verpoorte E. An integrated fritless column for on-chip capillary electrochromatography with conventional stationary phases. Anal Chem. 2002;74:639–47.
Maiolica A, Borsotti D, Rappsilber J. Self-made frits for nanoscale columns in proteomics. Proteomics. 2005;5:3847–50.
Banerjee S, Mazumdar S. Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte. Int J Anal Chem. 2012;40
Roberts LD, Virtue S, Vidal-Puig A, Nicholls AW, Griffin JL. Metabolic phenotyping of a model of adipocyte differentiation. Physiol Genomics. 2009;39:109–19.
Bollinger JG, Rohan G, Sadilek M, Gelb MH. LC/ESI-MS/MS detection of FAs by charge reversal derivatization with more than four orders of magnitude improvement in sensitivity. J Lipid Res. 2013;54:3523–30.
Murphy RC. Fatty acids. Tandem mass spectrometry of lipids: molecular analysis of complex lipids. The Royal Society of Chemistry; 2015. p. 1–39.
Rosenstock M, Greenberg AS, Rudich A. Distinct long-term regulation of glycerol and non-esterified fatty acid release by insulin and TNF-α in 3T3-L1 adipocytes. Diabetologia. 2001;44:55–62.
Zhou D, Samovski D, Okunade AL, Stahl PD, Abumrad NA, Su X. CD36 level and trafficking are determinants of lipolysis in adipocytes. FASEB J. 2012;26:4733–42.
Wang ZG, Pini M, Yao T, Zhou ZX, Sun CH, Fantuzzi G, et al. Homocysteine suppresses lipolysis in adipocytes by activating the AMPK pathway. Am J Physiol Endocrinol Metab. 2011;301:E703–12.
Acknowledgments
This research was funded by NIH R37 DK046960 to RTK. JPG was supported by NIH F32 EB019800. Spray tips and PicoClear unions were generously provided by New Objective Inc. TOF-MS experiments utilized Core Services of the Michigan Regional Comprehensive Metabolomics Resource Core (MRC2) supported by grant DK097153 of NIH to the University of Michigan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dugan, C.E., Grinias, J.P., Parlee, S.D. et al. Monitoring cell secretions on microfluidic chips using solid-phase extraction with mass spectrometry. Anal Bioanal Chem 409, 169–178 (2017). https://doi.org/10.1007/s00216-016-9983-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9983-0