Abstract
In this work, the bifunctional TiO2@SiO2-B(OH)2@Fe3O4@TiO2 sandwich-like nanosheets were designed and synthesized for the sequential selective enrichment of phosphopeptides and glycopeptides. Due to the bifunctional property of the titanium dioxide and the boronic acid group, the nanosheets were successfully applied to the enrichment of phosphopeptides and glycopeptides sequentially, evaluated by capturing phosphopeptides from tryptic digestion of model phosphoprotein bovine β-casein diluted to 0.02 ng/μL (8 × 10−16 mol/μL) and glycopeptides from tryptic digestion of model glycoprotein horseradish peroxidase (HRP) diluted to 0.1 ng/μL (2.5 × 10−15 mol/μL). The enrichment selectivity of the bifunctional nanosheets was evaluated by capturing phosphopeptides from a peptide mixture of β-casein and bovine serum albumin (BSA) with the molar ratio of 1:1000 (8.3 × 10−12 mol of β-casein and 8.3 × 10−9 mol of BSA in 100 μL) and glycopeptides from a peptide mixture of HRP and BSA up to the ratio of 1:50 (5.0 × 10−11 mol of HRP and 2.5 × 10−9 mol of BSA in 100 μL).

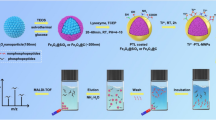
A workflow of the sequential enrichment strategy for phosphopeptides and glycopeptides by the bifunctional TiO2@SiO2-B(OH)2@Fe3O4@TiO2 sandwich-like nanosheets











Similar content being viewed by others
References
Pawson T, Scott JD. Protein phosphorylation in signaling—50 years and counting. Trends Biochem Sci. 2005;30(6):286–90.
Dennis JW, Nabi IR, Demetriou M. Metabolism, cell surface organization, and disease. Cell. 2009;139(7):1229–41.
Mann M, Ong SE, Grønborg M, Steen H, Jensen ON, Pandey A. Analysis of protein phosphorylation using mass spectrometry: deciphering the phosphoproteome. Trends Biotechnol. 2002;20(6):261–8.
Alvarez-Manilla G, Atwood III J, Guo Y, Warren NL, Orlando R, Pierce M. Tools for glycoproteomic analysis: size exclusion chromatography facilitates identification of tryptic glycopeptides with N-linked glycosylation sites. J Proteome Res. 2006;5(3):701–8.
Donnelly E, Goldstein I. Glutaraldehyde-insolubilized concanavalin A: an adsorbent for the specific isolation of polysaccharides and glycoproteins. Biochem J. 1970;118(4):679–80.
Drake PM, Schilling B, Niles RK, Braten M, Johansen E, Liu HC, et al. A lectin affinity workflow targeting glycosite-specific, cancer-related carbohydrate structures in trypsin-digested human plasma. Anal Biochem. 2011;408(1):71–85.
Hägglund P, Bunkenborg J, Elortza F, Jensen ON, Roepstorff P. A new strategy for identification of N-glycosylated proteins and unambiguous assignment of their glycosylation sites using HILIC enrichment and partial deglycosylation. J Proteome Res. 2004;3(3):556–66.
Sparbier K, Koch S, Kessler I, Wenzel T, Kostrzewa M. Selective isolation of glycoproteins and glycopeptides for MALDI-TOF MS detection supported by magnetic particles. J Biomol Tech. 2005;16(4):407–13.
Zhou HL, Watts JD, Aebersold R. A systematic approach to the analysis of protein phosphorylation. Nat Biotechnol. 2001;19(4):375–8.
McLachlin DT, Chait BT. Improved β-elimination-based affinity purification strategy for enrichment of phosphopeptides. Anal Chem. 2003;75(24):6826–36.
Ballif BA, Villén J, Beausoleil SA, Schwartz D, Gygi SP. Phosphoproteomic analysis of the developing mouse brain. Mol Cell Proteomics. 2004;3(11):1093–101.
Grønborg M, Kristiansen TZ, Stensballe A, Andersen JS, Ohara O, Mann M, et al. A mass spectrometry-based proteomic approach for identification of serine/threonine-phosphorylated proteins by enrichment with phospho-specific antibodies identification of a novel protein, Frigg, as a protein kinase A substrate. Mol Cell Proteomics. 2002;1(7):517–27.
Thingholm TE, Jørgensen TJ, Jensen ON, Larsen MR. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat Protoc. 2006;1(4):1929–35.
Nelson CA, Szczech JR, Xu QG, Lawrence MJ, Jin S, Ge Y. Mesoporous zirconium oxide nanomaterials effectively enrich phosphopeptides for mass spectrometry-based phosphoproteomics. Chem Commun. 2009;43:6607–9.
Gruhler A, Olsen JV, Mohammed S, Mortensen P, Færgeman NJ, Mann M, et al. Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway. Mol Cell Proteomics. 2005;4(3):310–27.
Sun ZY, Hamilton KL, Reardon KF. Evaluation of quantitative performance of sequential immobilized metal affinity chromatographic enrichment for phosphopeptides. Anal Biochem. 2014;445:30–7.
Tsai CF, Hsu CC, Hung JN, Wang YT, Choong WK, Zeng MY, et al. Sequential phosphoproteomic enrichment through complementary metal-directed immobilized metal ion affinity chromatography. Anal Chem. 2014;86(1):685–93.
Xu YW, Wu ZX, Zhang LJ, Lu HJ, Yang PY, Webley PA, et al. Highly specific enrichment of glycopeptides using boronic acid-functionalized mesoporous silica. Anal Chem. 2008;81(1):503–8.
Nelson CA, Szczech JR, Dooley CJ, Xu QG, Lawrence MJ, Zhu HY, et al. Effective enrichment and mass spectrometry analysis of phosphopeptides using mesoporous metal oxide nanomaterials. Anal Chem. 2010;82(17):7193–201.
Lu ZD, Duan JC, He L, Hu YX, Yin YD. Mesoporous TiO2 nanocrystal clusters for selective enrichment of phosphopeptides. Anal Chem. 2010;82(17):7249–58.
Wang JX, Wang YN, Gao MX, Zhang XM, Yang PY. Multilayer hydrophilic poly(phenol-formaldehyde resin)-coated magnetic graphene for boronic acid immobilization as a novel matrix for glycoproteome analysis. ACS Appl Mater Interfaces. 2015;7(29):16011–7.
Wang YL, Liu MB, Xie LQ, Fang CY, Xiong HM, Lu HJ. Highly efficient enrichment method for glycopeptide analyses: using specific and nonspecific nanoparticles synergistically. Anal Chem. 2014;86(4):2057–64.
Liu LT, Zhang Y, Zhang L, Yan GQ, Yao J, Yang PY, et al. Highly specific revelation of rat serum glycopeptidome by boronic acid-functionalized mesoporous silica. Anal Chim Acta. 2012;753:64–72.
Chen HM, Deng CH, Zhang XM. Synthesis of Fe3O4@ SiO2@ PMMA core-shell-shell magnetic microspheres for highly efficient enrichment of peptides and proteins for MALDI‐ToF MS analysis. Angew Chem Int Ed. 2010;49(3):607–11.
Hu LH, Zhou HJ, Li YH, Sun ST, Guo LH, Ye ML, et al. Profiling of endogenous serum phosphorylated peptides by titanium (IV) immobilized mesoporous silica particles enrichment and MALDI-TOFMS detection. Anal Chem. 2009;81(1):94–104.
Iorgulescu G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J Med Life. 2008;2(3):303–7.
Dodds MW, Johnson DA, Yeh CK. Health benefits of saliva: a review. J Dent. 2005;33(3):223–33.
Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent. 2009;22(4):241–8.
Sun NR, Deng CH, Li Y, Zhang XM. Size-exclusive magnetic graphene/mesoporous silica composites with titanium(IV)-immobilized pore walls for selective enrichment of endogenous phosphorylated peptides. ACS Appl Mater Interfaces. 2014;6(14):11799–804.
Acknowledgments
This work was supported by National Science Foundation of China (No. 21175026) and National Research Projects (2012YQ12004409, 2012CB910604, and 2013CB911201).
Authors’ contributions
All authors have given approval of the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The research followed the tenets of the Declaration of Helsinki, and the use of the human serum and saliva samples for research was approved by the Ethics Committee of Zhongshan Hospital, Fudan University. All individual participants gave informed consent for the use of these samples.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 794 kb)
Rights and permissions
About this article
Cite this article
Xu, D., Gao, M., Deng, C. et al. Synthesis of bifunctional TiO2@SiO2-B(OH)2@Fe3O4@TiO2 sandwich-like nanosheets for sequential selective enrichment of phosphopeptides and glycopeptides for mass spectrometric analysis. Anal Bioanal Chem 408, 5489–5497 (2016). https://doi.org/10.1007/s00216-016-9647-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9647-0