Abstract
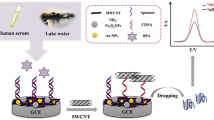
To specifically and sensitively identify bisphenol A (BPA) with a simple and rapid method is very important for food safety. Using an anti-BPA aptamer and Mo2C nanotubes, we developed a label-free and low-background signal biosensor for BPA detection. The anti-BPA aptamer drastically increased the fluorescence signal of N-methylmesoporphyrin IX under an assistance of Help-DNA. Additionally, BPA can interact with the anti-BPA aptamer and switch its conformation to prevent the formation of a G-quadruplex, resulting in fluorescence quenching. Simultaneously, Mo2C nanotubes can reduce the background signals due to the adsorption of Help-DNA on their surface. This method shows a linear range of 2–20 nM with a detection limit of 2 nM for detecting BPA. This label-free BPA aptasensor with low background signal is inexpensive, easy to use, and can be applied to determine BPA in real water samples.

A low-background and label-free biosensor was designed based on Mo2C nanotubes and aptamer for BPA detection.






Similar content being viewed by others
References
Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95.
Cui H, Wu J, Eda S, Chen J, Chen W, Zheng L. Rapid capacitive detection of femtomolar levels of bisphenol A using an aptamer-modified disposable microelectrode array. Microchim Acta. 2015;182:2361–7.
Xue F, Wu J, Chu H, Mei Z, Ye Y, Liu J, et al. Electrochemical aptasensor for the determination of bisphenol A in drinking water. Microchim Acta. 2013;180:109–15.
Lee JS, Kim SG, Jun J, Shin DH, Jang J. Aptamer-functionalized multidimensional conducting-polymer nanoparticles for an ultrasensitive and selective field-effect-transistor endocrine-disruptor sensors. Adv Funct Mater. 2014;24:6145–53.
Rochester JR. Bisphenol A, and human health: a review of the literature. Reprod Toxicol. 2013;42:132–55.
Chung E, Jeon J, Yu J, Lee C, Choo J. Surface-enhanced Raman scattering aptasensor for ultrasensitive trace analysis of bisphenol A. Biosens Bioelectron. 2015;64:560–5.
Xu J, Li Y, Bie J, Jiang W, Guo J, Luo Y, et al. Colorimetric method for determination of bisphenol A based on aptamer-mediated aggregation of positively charged gold nanoparticles. Microchim Acta. 2015;182:2131–8.
Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012;109:17525–30.
Zhou L, Wang J, Li D, Li Y. An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free detection of bisphenol A in milk samples. Food Chem. 2014;162:34–40.
Yoon Y, Westerhoff P, Snyder SA, Esparza M. HPLC-fluorescence detection and adsorption of bisphenol A, 17 beta-estradiol, and 17 alpha-ethynyl estradiol on powdered activated carbon. Water Res. 2003;37:3530–7.
Zafra-Gómez A, Ballesteros O, Navalón A, Vílchez JL. Determination of some endocrine disrupter chemicals in urban wastewater samples using liquid chromatography-mass spectrometry. Microchem J. 2008;88:87–94.
Kawaguchi M, Inoue K, Yoshimura M, Ito R, Sakui N, Okanouchi N, et al. Determination of bisphenol A in river water and body fluid samples by stir bar sorptive extraction with in situ derivatization and thermal desorption-gas chromatography–mass spectrometry. J Chromatogr B. 2004;805:41–8.
Zhao M-P, Li Y-Z, Guo Z-Q, Zhang X-X, Chang W-B. A new competitive enzyme-linked immunosorbent assay (ELISA) for determination of estrogenic bisphenols. Talanta. 2002;57:1205–10.
Watabe Y, Kondo T, Morita M, Tanaka N, Haginaka J, Hosoya K. Determination of bisphenol A in environmental water at ultra-low level by high-performance liquid chromatography with an effective on-line pretreatment device. J Chromatogr A. 2004;1032:45–9.
Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cienc Saude Coletiva. 2012;17:407–34.
Chen J, Duncan B, Wang Z, Wang LS, Rotello VM, Nugen SR. Bacteriophage-based nanoprobes for rapid bacteria separation. Nanoscale. 2015;7:16230–6.
Chen J, Li Y, Huang K, Wang P, He L, Carter KR, et al. Nanoimprinted patterned pillar substrates for surface-enhanced Raman scattering applications. ACS Appl Mater Interfaces. 2015;7:22106–13.
Zhang Y, Cao T, Huang X, Liu M, Shi H, Zhao G. A visible-light driven photoelectrochemical aptasensor for endocrine disrupting chemicals bisphenol A with high sensitivity and specificity. Electroanalysis. 2013;25:1787–95.
Yu P, Liu Y, Zhang X, Zhou J, Xiong E, Li X, et al. A novel electrochemical aptasensor for bisphenol A assay based on triple-signaling strategy. Biosens Bioelectron. 2016;79:22–8.
Long F, Zhu A, Shi H, Wang H. Hapten-grafted graphene as a transducer for homogeneous competitive immunoassay of small molecules. Anal Chem. 2014;86:2862–6.
Duan N, Zhang H, Nie Y, Wu S, Miao T, Chen J, et al. Fluorescence resonance energy transfer-based aptamer biosensors for bisphenol A using lanthanide-doped KGdF4 nanoparticles. Anal Methods. 2015;7:5186–92.
Wang K, Fan D, Liu Y, Wang E. Highly sensitive and specific colorimetric detection of cancer cells via dual-aptamer target binding strategy. Biosens Bioelectron. 2015;73:1–6.
Zhang D, Yang J, Ye J, Xu L, Xu H, Zhan S, et al. Colorimetric detection of bisphenol A based on unmodified aptamer and cationic polymer aggregated gold nanoparticles. Anal Biochem. 2016;499:51–6.
Zhang Y, Li B, Yan C, Fu L. One-pot fluorescence detection of multiple analytes in homogenous solution based on noncovalent assembly of single-walled carbon nanotubes and aptamers. Biosens Bioelectron. 2011;26:3505–10.
Deng W, Shen L, Wang X, Yang C, Yu J, Yan M, et al. Using carbon nanotubes-gold nanocomposites to quench energy from pinnate titanium dioxide nanorods array for signal-on photoelectrochemical aptasensing. Biosens Bioelectron. 2016;82:132–9.
Wang K, Ren J, Fan D, Liu Y, Wang E. Integration of graphene oxide and DNA as a universal platform for multiple arithmetic logic units. Chem Commun. 2014;50:14390–3.
Zhu Y, Cai Y, Xu L, Zheng L, Wang L, Qi B, et al. Building an aptamer/graphene oxide FRET biosensor for one-step detection of bisphenol A. ACS Appl Mater Interfaces. 2015;7:7492–6.
Liu JM, Yan XP. Competitive aptamer bioassay for selective detection of adenosine triphosphate based on metal-paired molecular conformational switch and fluorescent gold nanoclusters. Biosens Bioelectron. 2012;36:135–41.
Kuang H, Yin H, Liu L, Xu L, Ma W, Xu C. Asymmetric plasmonic aptasensor for sensitive detection of bisphenol A. ACS Appl Mater Interfaces. 2014;6:364–9.
Chen WF, Wang CH, Sasaki K, Marinkovic N, Xu W, Muckerman JT, et al. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ Sci. 2013;6:943–51.
Ma FX, Wu HB, Xia BY, Xu CY, Lou XW. Hierarchical beta-Mo2C nanotubes organized by ultrathin nanosheets as a highly efficient electrocatalyst for hydrogen production. Angew Chem Int Ed. 2015;54:15395–9.
Xiao P, Ge X, Wang H, Liu Z, Fisher A, Wang X. Novel molybdenum carbide-tungsten carbide composite nanowires and their electrochemical activation for efficient and stable hydrogen evolution. Adv Funct Mater. 2015;25:1520–6.
Vrubel H, Hu X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew Chem Int Ed. 2012;51:12703–6.
Xing Z, Wang L, Sun X, He Y, Asiri AM. Nanoporous molybdenum carbide nanowires: a novel sensing platform for DNA detection. J Mater Chem B. 2015;3:7173–6.
Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc Natl Acad Sci U S A. 2002;99:11593–8.
Oh SS, Plakos K, Lou XH, Xiao Y, Soh HT. In vitro selection of structure-switching. Self-reporting aptamers. Proc Natl Acad Sci U S A. 2010;107:14053–8.
Jo M, Ahn JY, Lee J, Lee S, Hong SW, Yoo JW, et al. Development of single-stranded DNA aptamers for specific bisphenol A detection. Oligonucleotides. 2011;21:85–91.
Marks HL, Pishko MV, Jackson GW, Cote GL. Rational design of a bisphenol A aptamer selective surface-enhanced Raman scattering nanoprobe. Anal Chem. 2014;86:11614–9.
Yildirim N, Long F, He M, Shi HC, Gu AZ. A portable optic fiber aptasensor for sensitive, specific and rapid detection of bisphenol-A in water samples. Environ Sci Process Impacts. 2014;16:1379–86.
Acknowledgements
This work was financially supported by the Natural Science Foundation of China (51572044, 21475018), the Program of New Century Excellent Talents in University (NCET-13-0114), and the Fundamental Research Funds for the Central Universities (N140506001, N140505005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human participants and/or animals
This work does not contain any studied with human participants or animals.
Additional information
Meng-Qi He and Kun Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 475 kb)
Rights and permissions
About this article
Cite this article
He, MQ., Wang, K., Wang, J. et al. A sensitive aptasensor based on molybdenum carbide nanotubes and label-free aptamer for detection of bisphenol A. Anal Bioanal Chem 409, 1797–1803 (2017). https://doi.org/10.1007/s00216-016-0123-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0123-7