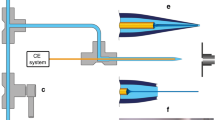
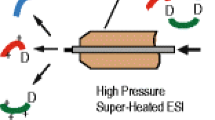
Abstract
Capillary zone electrophoresis-electrospray ionization-mass spectrometry (CZE-ESI-MS) is attracting renewed attention for proteomic and metabolomic analysis. An important reason for this interest is the maturation and commercialization of interfaces for coupling CZE with ESI-MS. One of these interfaces is an electro-kinetically pumped sheath flow nanospray interface developed by the Dovichi group, in which a very low sheath flow is generated based on electroosmosis within a glass emitter. CMP Scientific has commercialized this interface as the EMASS-II ion source. In this work, we compared the performance of the EMASS-II ion source with our in-house system. The performance of the systems is equivalent. We also coupled the EMASS-II ion source with a PrinCE Next|480 capillary electrophoresis autosampler and an Orbitrap mass spectrometer, and analyzed this system’s performance in terms of sensitivity, reproducibility, and separation performance for separation of tryptic digests, intact proteins, and amino acids. The system produced reproducible analysis of BSA digest; the RSDs of peptide intensity and migration time across 24 runs were less than 20 and 6%, respectively. The system produced a linear calibration curve of intensity across a 30-fold range of tryptic digest concentration. The combination of a commercial autosampler and electrospray interface efficiently separated amino acids, peptides, and intact proteins, and only required 5 μL of sample for analysis.

The commercial and locally constructed versions of the interface provide similar numbers of protein identifications from a Xenopus laevis fertilized egg digest






Similar content being viewed by others
References
Sun L, Zhu G, Yan X, et al. Capillary zone electrophoresis for bottom-up analysis of complex proteomes. Proteomics. 2016;16:188–96.
Hirayama A, Wakayama M, Soga T. Metabolome analysis based on capillary electrophoresis-mass spectrometry. TrAC Trends Anal Chem. 2014;61:215–22.
Ramautar R, Somsen GW, de Jong GJ. CE-MS for metabolomics: developments and applications in the period 2012–2014. Electrophoresis. 2015;36:212–24.
Maxwell EJ, Chen DDY. Twenty years of interface development for capillary electrophoresis-electrospray ionization-mass spectrometry. Anal Chim Acta. 2008;627:25–33.
Smith RD, Barinaga CJ, Udseth HR. Improved electrospray ionization interface for capillary zone-mass spectrometry. Anal Chem. 1988;60:1948–52.
Whitt JT, Moini M. Capillary electrophoresis to mass spectrometry interface using a porous junction. Anal Chem. 2003;75:2188–91.
Wojcik R, Dada OO, Sadilek M, Dovichi NJ. Simplified capillary electrophoresis nanospray sheath-flow interface for high efficiency and sensitive peptide analysis. Rapid Commun Mass Spectrom. 2010;24:2554–60.
Sun L, Zhu G, Zhang Z, Mou S, Dovichi NJ. Third-generation electrokinetically pumped sheath-flow nanospray interface with improved stability and sensitivity for automated capillary zone electrophoresis–mass spectrometry analysis of complex proteome digests. J Proteome Res. 2015;14:2312–21.
Sun L, Hebert AS, Yan X, et al. Over 10000 peptide identifications from the hela proteome by using single-shot capillary zone electrophoresis combined with tandem mass spectrometry. Angew Chemie Int Ed. 2014;53:13931–3.
Zhao Y, Riley NM, Sun L, et al. Coupling capillary zone electrophoresis with electron transfer dissociation and activated ion electron transfer dissociation for top-down proteomics. Anal Chem. 2015;87:5422–9.
Zhao Y, Sun L, Knierman MD, Dovichi NJ. Fast separation and analysis of reduced monoclonal antibodies with capillary zone electrophoresis coupled to mass spectrometry. Talanta. 2016;148:529–33.
Schiavone NM, Sarver SA, Sun L, Wojcik R, Dovichi NJ. High speed capillary zone electrophoresis-mass spectrometry via an electrokinetically pumped sheath flow interface for rapid analysis of amino acids and a protein digest. J Chromatogr B Anal Technol Biomed Life Sci. 2015;991:53–8.
Han M, Rock BM, Pearson JT, Rock DA. Intact mass analysis of monoclonal antibodies by capillary electrophoresis—mass spectrometry. J Chromatogr B. 2016;1011:24–32.
Sun X, Lin L, Liu X, et al. Capillary electrophoresis–mass spectrometry for the analysis of heparin oligosaccharides and low molecular weight heparin. Anal Chem. 2016;88:1937–43.
Peuchen EH, Sun L, Dovichi NJ. Optimization and comparison of bottom-up proteomic sample preparation for early-stage Xenopus laevis embryos. Anal Bioanal Chem. 2016;408:4743–9.
Sun L, Zhu G, Zhao Y, Yan X, Mou S, Dovichi NJ. Ultrasensitive and fast bottom-up analysis of femtogram amounts of complex proteome digests. Angew Chem Int Ed Engl. 2013;52:13661–4.
Zhu G, Sun L, Dovichi NJ. Thermally-initiated free radical polymerization for reproducible production of stable linear polyacrylamide coated capillaries, and their application to proteomic analysis using capillary zone electrophoresis–mass spectrometry. Talanta. 2016;146:839–43.
Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–72.
Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794–805.
Wühr M, Freeman RM, Presler M, et al. Deep proteomics of the Xenopus laevis egg using an mRNA-derived reference database. Curr Biol. 2014;24:1467–75.
Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13:2513–26.
Acknowledgments
We give a special thanks to Prince Technologies, especially the Service Department (Sean Katuin and Harry Buning), for their partnership and troubleshooting expertise during experiment design and testing. We also give a special thanks to CMP Scientific, Corp. for providing the EMASS-II CE-MS interface for the experiments. We thank Drs. William Boggess and Matthew Champion in the Notre Dame Mass Spectrometry and Proteomics Facility for their help with this project. This project was supported by a grant from the National Institutes of Health (R01GM096767). EHP acknowledges support from the National Science Foundation Graduate Research Fellowship program (2015–2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Norman J. Dovichi, Liangliang Sun, and Guije Zhu are co-inventors on patents describing the electrospray interface and receive royalties on the sale of the CMP interface. Elizabeth H. Peuchen declares no conflict of interest.
Research involving animals
All animal procedures were performed according to protocols approved by the University of Notre Dame Institutional Animal Care and Use Committee.
Rights and permissions
About this article
Cite this article
Peuchen, E.H., Zhu, G., Sun, L. et al. Evaluation of a commercial electro-kinetically pumped sheath-flow nanospray interface coupled to an automated capillary zone electrophoresis system. Anal Bioanal Chem 409, 1789–1795 (2017). https://doi.org/10.1007/s00216-016-0122-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0122-8