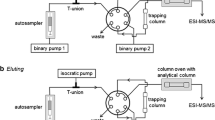
Abstract
A molecularly imprinted polymer (MIP) selective for cannabinoids [Δ9-tetrahydrocannabinol (Δ9-THC), 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (Δ9-THC-COOH), and 11-hydroxy-Δ9-tetrahydrocannabinol (Δ9-THC-OH)] has been synthesized, fully characterized, and applied to the assessment of plasma and urine analysis of marijuana abuse by high performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS). Δ9-THC-COOH was used as a template molecule, whereas ethylene glycol dimethacrylate (EGDMA) was used as a functional monomer, divinylbenzene (DVB) as a cross-linker, and 2,2′-azobisisobutyronitrile (AIBN) as an initiator. The prepared MIP was found to be highly selective for cannabinoids typically found in blood and urine, and also for cannabinol (CBN) and cannabidiol (CBD). MIP beads (50 mg) were loaded inside a cone-shaped device made of a polypropylene (PP) membrane for microsolid-phase extraction (μ-SPE) in batch mode. Optimum retention of analytes (0.1 to 1.0 mL of plasma/urine) was achieved by fixing plasma/urine pH at 6.5 and assisting the procedure by mechanical shaking (150 rpm, 40 °C, 12 min). Optimum elution conditions implied 2 mL of a 90:10 methanol/acetic acid and ultrasound extraction (35 kHz, 325 W) for 6 min. Good precision was assessed by intra-day and inter-day assays. In addition, the method was found to be accurate after intra-day and inter-day analytical recovery assays and after analyzing control serum and urine control samples. The limits of quantification were in the range of 0.36–0.49 ng L−1 (plasma analysis) and 0.47–0.57 ng L−1 (urine analysis). These values are low enough for confirmative conclusions regarding marijuana abuse through blood and urine analysis.

ᅟ







Similar content being viewed by others
References
European Monitoring Centre for Drug and Drug Addiction. European Drug Report—trends and developments 2015. Available at: http://www.emcdda.europa.eu/attachements.cfm/att_239505_EN_TDAT15001ENN.pdf. Accessed March 30th 2016.
Teixeira H, Verstraete A, Proenc P¸ Corte-Real F, Monsanto P, Vieira DN. Validated method for the simultaneous determination of Δ9-THC and Δ9-THC-COOH in oral fluid, urine and whole blood using solid-phase extraction and liquid chromatography–mass spectrometry with electrospray ionization. Forensic Sci Int. 2007;170:148–55.
Raharjo TJ, Verpoorte R. Methods for the analysis of cannabinoids in biological materials: a review. Phytochem Anal. 2004;15:79–94.
König S, Aebi B, Lanz S, Gasser M, Weinmann W. On-line SPE LC-MS/MS for the quantification of Δ9-tetrahydrocannabinol (THC) and its two major metabolites in human peripheral blood by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2011;400:9–16.
Sadler Simões S, Castañera Ajenjo A, Dias MJ. Qualitative and quantitative analysis of THC, 11-hydroxy-THC and 11-nor-9-carboxy-THC in whole blood by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2011;25:2603–10.
Fabritius M, Chtioui H, Battistella G, Annoni J-M, Dao K, Favrat B, et al. Comparison of cannabinoid concentrations in oral fluid and whole blood between occasional and regular cannabis smokers prior to and after smoking a cannabis joint. Anal Bioanal Chem. 2013;405:9791–803.
Ferreirós N, Labocha S, Walter C, Lötsch J, Geisslinger G. Simultaneous and sensitive LC–MS/MS determination of tetrahydrocannabinol and metabolites in human plasma. Anal Bioanal Chem. 2013;405:1399–406.
Sergi M, Battista N, Montesano C, Curini R, Maccarrone M, Compagnone D. Determination of the two major endocannabinoids in human plasma by μ-SPE followed by HPLC-MS/MS. Anal Bioanal Chem. 2013;405:785–93.
Mercolini L, Mandrioli R, Sorella V, Somaini L, Giocondi D, Serpelloni G, et al. Dried blood spots: liquid chromatography–mass spectrometry analysis of _9-tetrahydrocannabinol and its main metabolites. J Chromatogr A. 2013;1271:33–40.
Hidvégi E, Somogyi GP. Determination of main tetrahydrocannabinoids by GC-MS: impact of protein precipitation by acetonitrile on solid phase extraction of cannabinoids from human serum. Pharmazie. 2014;69:417–9.
Toennes SW, Hanisch S, Pogoda W, Wunder C, Paulke A. Pitfall in cannabinoid analysis—detection of a previously unrecognized interfering compound in human serum. Anal Bioanal Chem. 2015;407:463–70.
Basheer C, Alnedhary AA, Madhava Rao BS, Valliyaveettil S, Lee HK. Development and application of porous membrane-protected carbon nanotube micro-solid-phase extraction combined with gas chromatography/mass spectrometry. Anal Chem. 2006;78:2853–8.
Basheer C, Chong HG, Hii TM, Lee HK. Application of porous membrane-protected micro-solid-phase extraction combined with HPLC for the analysis of acidic drugs in waste water. Anal Chem. 2007;79:6845–50.
Basheer C, Alnedhary AA, Madhava Rao BS, Lee HK. Determination of carbamate pesticides using micro-solid-phase extraction combined with high-performance liquid chromatography. J Chromatogr A. 2009;1216:211–6.
Khayoon WS, Saad B, Salleh B, Manaf NHA, Latiff AA. Micro-solid phase extraction with liquid chromatography–tandem mass spectrometry for the determination of aflatoxins in coffee and malt beverage. Food Chem. 2014;147:287–94.
Kanimozhi S, Basheer C, Narasimhan K, Liu L, Koh S, Xue F, et al. Application of porous membrane protected micro-solid-phase-extraction combined with gas chromatography–mass spectrometry for the determination of estrogens in ovarian cyst fluid samples. Anal Chim Acta. 2011;687:56–60.
Nsubuga H, Basheer C. Determination of haloacetic acids in swimming pool waters by membrane-protected micro-solid phase extraction. J Chromatogr A. 2013;1315:47–52.
Lim TH, Hu L, Yang C, He C, Lee HK. Membrane assisted micro-solid phase extraction of pharmaceuticals with amino and urea-grafted silica gel. J Chromatogr A. 2013;1316:8–14.
Ge D, Lee HK. Water stability of zeolite imidazolate framework 8 and application to porous membrane-protected micro-solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J Chromatogr A. 2011;1218:8490–5.
Ge D, Lee HK. Sonication-assisted emulsification microextraction combined with vortex-assisted porous membrane-protected micro-solid-phase extraction using mixed zeolitic imidazolate frameworks 8 as sorbent. J Chromatogr A. 2012;1263:1–6.
Ge D, Lee HK. Zeolite imidazolate frameworks 8 as sorbent and its application to sonication-assisted emulsification microextraction combined with vortex-assisted porous membrane-protected micro-solid-phase extraction for fast analysis of acidic drugs in environmental water samples. J Chromatogr A. 2012;1257:19–24.
Ara KM, Pandidan S, Aliakbari A, Raofie F, Amini M. Porous-membrane-protected polyaniline-coated SBA-15 nanocomposite micro-solid-phase extraction followed by high-performance liquid chromatography for the determination of parabens in cosmetic products and wastewater. J Sep Sci. 2015;38:1213–24.
Wang T, Wang J, Zhang C, Yang Z, Dai X, Cheng M, et al. Metal-organic framework MIL-101(Cr) as a sorbent of porous membrane-protected micro-solid-phase extraction for the analysis of six phthalate esters from drinking water: a combination of experimental and computational study. Analyst. 2015;140:5308–16.
Chen L, Xu S, Li J. Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev. 2011;40:2922–42.
Martín-Esteban A. Molecularly-imprinted polymers as a versatile, highly selective tool in sample preparation. Trends Anal Chem. 2013;45:169–81.
Chen L, Wang X, Lu W, Wu X, Lia J. Molecular imprinting: perspectives and applications. Chem Soc Rev. 2016;45:2137–211.
Tan F, Deng M, Liu X, Zhao H, Li X, Quan X, et al. Evaluation of a novel microextraction technique for aqueous samples: porous membrane envelope filled with multiwalled carbon nanotubes coated with molecularly imprinted polymer. J Sep Sci. 2011;34:707–15.
Lee TP, Saad B, Khayoon WS, Salleh B. Molecularly imprinted polymer as sorbent in micro-solid phase extraction of ochratoxin A in coffee, grape juice and urine. Talanta. 2012;88:129–35.
Yin X-Y, Luo Y-M, Fu J, Zhong Y-Q, Liu Q-S. Determination of hyperoside and isoquercitrin in rat plasma by membrane protected micro-solid-phase extraction with high-performance liquid chromatography. J Sep Sci. 2012;35:384–91.
Sánchez-González J, Tabernero MJ, Bermejo AM, Bermejo-Barrera P, Moreda-Piñeiro A. Porous membrane-protected molecularly imprinted polymer microsolid-phase extraction for analysis of urinary cocaine and its metabolites using liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2015;898:50–9.
Sánchez-González J, García-Carballal S, Cabarcos P, Tabernero MJ, Bermejo-Barrera P, Moreda-Piñeiro A. Determination of cocaine and its metabolites in plasma by porous membrane-protected molecularly imprinted polymer micro-solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2016;1451:15–22.
Cela-Pérez MC, Bates F, Jiménez-Morigosa C, Lendoiro E, de Castro A, Cruz A, et al. Water-compatible imprinted pills for sensitive determination of cannabinoids in urine and oral fluid. J Chromatogr A. 2016;1429:53–64.
Sánchez-González J, Tabernero MJ, Bermejo AM, Bermejo-Barrera P, Moreda-Piñeiro A. Development of magnetic molecularly imprinted polymers for solid phase extraction of cocaine and metabolites in urine before high performance liquid chromatography–tandem mass spectrometry. Talanta. 2016;147:641–9.
Sánchez-González J, Barreiro-Grille T, Cabarcos P, Tabernero MJ, Bermejo-Barrera P, Moreda-Piñeiro A. Magnetic molecularly imprinted polymer based–micro-solid phase extraction of cocaine and metabolites in plasma followed by high performance liquid chromatography–tandem mass spectrometry. Microchem J. 2016;127:206–12.
Chantada-Vázquez MP, Sánchez-González J, Peña-Vázquez E, Tabernero MJ, Bermejo AM, Bermejo-Barrera P, et al. Synthesis and characterization of novel molecularly imprinted polymer-coated Mn-doped ZnS quantum dots for specific fluorescent recognition of cocaine. Biosens Bioelectron. 2016;75:213–21.
Chantada-Vázquez MP, Sánchez-González J, Peña-Vázquez E, Tabernero MJ, Bermejo AM, Bermejo-Barrera P, et al. Simple and sensitive molecularly imprinted polymer-Mn-doped ZnS quantum dots based fluorescence probe for cocaine and metabolites determination in urine. Anal Chem. 2016;88:2734–41.
Wu X, Wang X, Lu W, Wang X, Li J, You H, et al. Water-compatible temperature and magnetic dual-responsive molecularly imprinted polymers for recognition and extraction of bisphenol A. J Chromatogr A. 2016;435:30–8.
Moreda-Piñeiro A, Moreda-Piñeiro J, Bermejo-Barrera P. Sample pre-treatment methods for organometallic species determination. In: Bakirdere S, editor. Speciation studies in soil, sediment and environmental samples. Boca Raton: CRC Press Taylor & Francis Group; 2014. p. 19–201.
Basheer C, Jayaraman A, Kee MK, Valiyaveettil S, Lee HK. Polymer-coated hollow-fiber microextraction of estrogens in water samples with analysis by gas chromatography–mass spectrometry. J Chromatogr A. 2005;1100:137–43.
Basheer C, Lee J, Pedersen-Bjergaard S, Rasmussen KE, Lee HK. Simultaneous extraction of acidic and basic drugs at neutral sample pH: a novel electro-mediated microextraction approach. J Chromatogr A. 2010;1217:6661–7.
Vindenes V, Lund HME, Andresen W, Gjerde H, Ikdahl SE, Christophersen AS, et al. Detection of drugs of abuse in simultaneously collected oral fluid, urine and blood from Norwegian drug drivers. Forensic Sci Int. 2012;219:165–71.
Horwitz W. Evaluation of analytical methods used for regulation of foods and drugs. Anal Chem. 1982;54:67A–76A.
Baselt RC. Disposition of toxic drugs and chemicals in man. 9th edition. Seal Beach: Biomedical Publications; 2011. p. 1644–8.
Acknowledgments
The authors wish to thank the Dirección Xeral de I+D – Xunta de Galicia (Project number 10CSA209042PR) and the European Regional Development Funds 2007-2013 (FEDER), Infrastructure Program UNST-10-1E-1195 (Ministerio de Economía y Competitividad, Spain) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that the studies have been approved by the Comité Ético de Investigación Clínica de Galicia (registration code CEIC de Galicia 2010/372).
The approved document by the CEIC de Galicia requires an informed consent from all volunteers who participated in the study. The authors declare therefore that all volunteers have signed an informed consent for allowing the use of the provided blood and urine samples in this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 805 kb)
Rights and permissions
About this article
Cite this article
Sánchez-González, J., Salgueiro-Fernández, R., Cabarcos, P. et al. Cannabinoids assessment in plasma and urine by high performance liquid chromatography–tandem mass spectrometry after molecularly imprinted polymer microsolid-phase extraction. Anal Bioanal Chem 409, 1207–1220 (2017). https://doi.org/10.1007/s00216-016-0046-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0046-3