Abstract
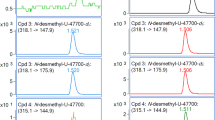
A new designer drug, a dissociative anesthetic, and a putative N-methyl-d-aspartate receptor antagonist, methoxetamine (MXE) noted by the EU Early Warning System has been already identified as a cause of several fatalities worldwide. The primary objective of this work was to develop a suitable sample preparation method allowing for isolation of MXE and its main metabolites in high yields from rat brain, liver, and lungs. For the purpose of the project, MXE and five metabolites were synthesized in-house, specifically O-desmethyl-normethoxetamine, O-desmethylmethoxetamine, dihydro-O-desmethylmethoxetamine, normethoxetamine, and dihydromethoxetamine. A sample preparation procedure consisted in the homogenization of the tissue applying salting-out-assisted liquid–liquid extraction (SALLE). A subsequent liquid chromatography-mass spectrometry (LC-MS) analysis was based on reversed-phased chromatography hyphenated with a triple quad MS system in a positive electrospray mode. Multiple reaction monitoring (MRM) was used for qualification and quantification of the analytes. The quantification was based on the application of an isotopically labeled internal standard, normethoxetamine-d3. The matrix-matched calibrations were prepared for each type of matrix with regression coefficients 0.9943–1.0000. The calibration curves were linear in the concentration range of 2.5–250 ng g−1. Limits of quantification (LOQs) were estimated as 2.5 and 5 ng g−1, respectively. Recovery (80–117 %) and matrix effect (94–110 %) at 100 ng g−1 and intra- and inter-day accuracy and precision at low (2.5 ng g−1), middle (25 ng g−1), and upper (250 ng g−1) concentration levels for all the analytes in all three types of tissues were also determined. The developed analytical method was applied to a set of real samples gathered in toxicological trials on rats and MXE, and its metabolites were determined successfully.





Similar content being viewed by others
References
EMCDDA (2014) Methoxetamine—report on the risk assessment of 2-(3-methoxyphenyl)-2-(ethylamino)cyclohexanone (methoxetamine) in the framework of the Council Decision on new psychoactive substances, Lisbon
Kjellgren A, Jonsson K (2013) Methoxetamine (MXE)—a phenomenological study of experiences induced by a “legal high” from the internet. J Psychoactive Drugs 45:276–286
Meyer MR, Bach M, Welter J, Bovens M, Turcant A, Maurer HH (2013) Ketamine-derived designer drug methoxetamine: metabolism including isoenzyme kinetics and toxicological detectability using GC-MS and LC-(HR-)MSn. Anal Bioanal Chem 405:6307–6321
Corazza O, Assi S, Schifano F (2013) From “Special K” to “Special M”: the evolution of the recreational use of ketamine and methoxetamine. CNS Neurosci Ther 19:454–460
Shields JE, Dargan PI, Wood DM, Puchnarewicz M, Davies S, Waring WS (2012) Methoxetamine associated reversible cerebellar toxicity: three cases with analytical confirmation. Clin Toxicol 50:438–440
Wiergowski M, Anand JS, Krzyżanowski MJZ (2014) Acute methoxetamine and amphetamine poisoning with fatal outcome: a case report. Int J Occup Med Environ Health 27:683–690
Hays PA, Casale JF, Berrier AL (2012) The characterization of 2-(3-methoxyphenyl)-2-(ethylamino)cyclohexanone (Methoxetamine). Microchem J 9:3–17
Hydzik P, Gomółka E, Sulka A, Cudzich-czop S (2012) Acute methoxetamine poisoning—case report. 91:235–242
Loeffler G, Craig C (2013) Methoxetamine misuse and toxicity. J Stud Alcohol Drugs 74:816–817
De Paoli G, Brandt SD, Wallach J, Archer RP, Pounder DJ (2013) From the street to the laboratory: analytical profiles of methoxetamine, 3-methoxyeticyclidine and 3-methoxyphencyclidine and their determination in three biological matrices. J Anal Toxicol 37:277–283
Abe E, Ricard F, Darrouzain F, Alvarez JC (2013) An automated method for measurement of methoxetamine in human plasma by use of turbulent flow on-line extraction coupled with liquid chromatography and mass spectrometric detection. Anal Bioanal Chem 405:239–245
Zawilska JB (2014) Methoxetamine—a novel recreational drug with potent hallucinogenic properties. Toxicol Lett 230:402–407
Al-Saffar Y, Stephanson NN, Beck O (2013) Multicomponent LC-MS/MS screening method for detection of new psychoactive drugs, legal highs, in urine—experience from the Swedish population. J Chromatogr B Anal Technol Biomed Life Sci 930:112–120
Menzies EL, Hudson SC, Dargan PI, Parkin MC, Wood DM, Kicman AT (2014) Characterizing metabolites and potential metabolic pathways for the novel psychoactive substance methoxetamine. Drug Test Anal 6:506–515
Zhang J, Wu H, Kim E, El-Shourbagy TA (2009) Salting-out assisted liquid/liquid extraction with acetonitrile: a new high throughput sample preparation technique for good laboratory practice bioanalysis using liquid chromatography-mass spectrometry. Biomed Chromatogr 23:419–425
Liu J, Jiang M, Li G, Xu L, Xie M (2010) Miniaturized salting-out liquid-liquid extraction of sulfonamides from different matrices. Anal Chim Acta 679:74–80
Wu H, Zhang J, Norem K, El-Shourbagy TA (2008) Simultaneous determination of a hydrophobic drug candidate and its metabolite in human plasma with salting-out assisted liquid/liquid extraction using a mass spectrometry friendly salt. J Pharm Biomed Anal 48:1243–1248
Valente IM, Gonçalves LM, Rodrigues JA (2013) Another glimpse over the salting-out assisted liquid-liquid extraction in acetonitrile/water mixtures. J Chromatogr A 1308:58–62
Anastassiades M, Scherbaum E (2005) Chapter 4 sample handling and clean-up procedures II-new developments. Compr Anal Chem 43:113–233
Schenck FJ, Callery P, Gannett PM, Daft JR, Lehotay SJ (2002) Schenck.pdf. J AOAC Int 85:1177–1180
Guo Y-C, Li X-H, Zhao L-J, Zhang Y-H (2013) Drawing out the structural information about the first hydration layer of the isolated Cl− anion through the FTIR-ATR difference spectra. J Solut Chem 42:459–469
Acknowledgments
This study was funded by a specific university research grant (project MSMT No 20/2015) and by the Ministry of Interior of the Czech Republic (project VG20122015075, New synthetic drugs (NSD)—creation of toxicological database, development and validation of detection methods including fast immunochemical tests, behavioral pharmacology, pharmacokinetics and biotransformation in rats, epidemiology).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 635 kb)
Rights and permissions
About this article
Cite this article
Hajkova, K., Jurasek, B., Sykora, D. et al. Salting-out-assisted liquid–liquid extraction as a suitable approach for determination of methoxetamine in large sets of tissue samples. Anal Bioanal Chem 408, 1171–1181 (2016). https://doi.org/10.1007/s00216-015-9221-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9221-1