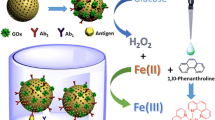
Abstract
A naked-eye sensitive ELISA-like assay was developed based on gold-enhanced peroxidase-like activity of gold nanoparticles (AuNPs). Using human IgG (H-IgG) as an analytical model, goat anti-human IgG antibody (anti-IgG) adsorbed on microtiter plate and AuNPs-labeled anti-IgG acted as capture antibody and detection antibody, respectively. Because the surfaces of AuNPs were blocked by protein molecules, the peroxidase-like activity of AuNPs was almost inhibited, evaluated by the catalytic oxidation of peroxidase enzyme substrate 3,3′,5,5′-tetramethylbenzidine (TMB), which could produce a bright blue color in the presence of H2O2. Fortunately, the catalytic ability of AuNPs was dramatically increased by the deposition of gold due to the formation of a new gold shell on immunogold. Under optimal reaction conditions, the colorimetric immunoassay presented a good linear relationship in the range of 0.7–100 ng/mL and the limit of detection (LOD) of 0.3 ng/mL calculated by 3σ/S for UV-vis detection, and obtained LOD of 5 ng/mL for naked-eye detection. The obtained results were competitive with conventional sandwich ELISA with the LOD of 1.6 ng/mL. Furthermore, this developed colorimetric immunoassay was successfully applied to diluted human serum and fetal bovine serum samples, and predicted a broad prospect for the use of peroxidase-like activity involving nanomaterials in bioassay and diagnostics.

A naked-eye ELISA-like immunoassay for determination of human IgG is described based on gold enhanced peroxidase like activity of gold nanoparticles and it is a colorimetric typical sandwich-type immunoassay format with high sensitivity



Similar content being viewed by others
References
Liu Z, Zhou B, Wang H, Lu F, Liu T, Song C, Leng X (2013) Highly sensitive detection of human IgG using a novel bio-barcode assay combined with DNA chip technology. J Nanopart Res 15(9):1–14
Balzerova A, Fargasova A, Markova Z, Ranc V, Zboril R (2014) Magnetically-assisted surface enhanced Raman spectroscopy (MA-SERS) for label-free determination of human immunoglobulin G (IgG) in blood using Fe3O4@Ag nanocomposite. Anal Chem 86(22):11107–11114
Engvall E, Perlmann P (1971) Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 8(9):871–874
Matthews GS, Miller K, Creminon C, Wal JM (1997) An enzyme-linked immunosorbent assay for the detection of antigen-specific rat immunoglobulin E with improved sensitivity upon a conventional horseradish peroxidase-based ELISA method. Biochem Soc Trans 25(2):S375–S375
Li D, Ying Y, Wu J, Niessner R, Knopp D (2013) Comparison of monomeric and polymeric horseradish peroxidase as labels in competitive ELISA for small molecule detection. Microchim Acta 180(7/8):711–717
Gao L, Wu J, Lyle S, Zehr K, Cao L, Gao D (2008) Magnetite nanoparticle-linked immunosorbent assay. J Phys Chem C 112(44):17357–17361
Lavery CB, MacInnis MC, MacDonald MJ, Williams JB, Spencer CA, Burke AA, Irwin DJG, D’Cunha GB (2010) Purification of peroxidase from horseradish (Armoracia rusticana) roots. J Agric Food Chem 58(15):8471–8476
Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M (2005) Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 21(23):10644–10654
Grabar KC, Freeman RG, Hommer MB, Natan MJ (1995) Preparation and characterization of Au colloid monolayers. Anal Chem 67(4):735–743
Mayer KM, Hafner JH (2011) Localized surface plasmon resonance sensors. Chem Rev 111(6):3828–3857
Wang S, Wang X, Zhang Z, Chen L (2015) Highly sensitive fluorescence detection of copper ion based on its catalytic oxidation to cysteine indicated by fluorescein isothiocyanate functionalized gold nanoparticles. Colloid Surf A Physicochem Eng Asp 468:333–338
Liu Y, Liu Y, Mernaugh RL, Zeng X (2009) Single chain fragment variable recombinant antibody functionalized gold nanoparticles for a highly sensitive colorimetric immunoassay. Biosens Bioelectron 24(9):2853–2857
Chen J, Huang Y, Zhao S, Lu X, Tian J (2012) Gold nanoparticles-based fluorescence resonance energy transfer for competitive immunoassay of biomolecules. Analyst 137(24):5885–5890
Wang Y, Tang L-J, Jiang J-H (2013) Surface-enhanced Raman spectroscopy-based, homogeneous, multiplexed immunoassay with antibody-fragments-decorated gold nanoparticles. Anal Chem 85(19):9213–9220
Yang X-Y, Guo Y-S, Bi S, Zhang S-S (2009) Ultrasensitive enhanced chemiluminescence enzyme immunoassay for the determination of α-fetoprotein amplified by double-codified gold nanoparticles labels. Biosens Bioelectron 24(8):2707–2711
Zhu Z, Shi L, Feng H, Susan Zhou H (2015) Single domain antibody coated gold nanoparticles as enhancer for Clostridium difficile toxin detection by electrochemical impedance immunosensors. Bioelectrochemistry 101:153–158
Zhou X, Cao P, Zhu Y, Lu W, Gu N, Mao C (2015) Phage-mediated counting by the naked eye of miRNA molecules at attomolar concentrations in a Petri dish. Nat Mater 14(10):1058–1064
Valentini P, Fiammengo R, Sabella S, Gariboldi M, Maiorano G, Cingolani R, Pompa PP (2013) Gold-nanoparticle-based colorimetric discrimination of cancer-related point mutations with picomolar sensitivity. ACS Nano 7(6):5530–5538
Jv Y, Li B, Cao R (2010) Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem Commun 46(42):8017–8019
Liu M, Zhao H, Chen S, Yu H, Quan X (2012) Interface engineering catalytic graphene for smart colorimetric biosensing. ACS Nano 6(4):3142–3151
Gao Z, Xu M, Lu M, Chen G, Tang D (2015) Urchin-like (gold core)@(platinum shell) nanohybrids: a highly efficient peroxidase-mimetic system for in situ amplified colorimetric immunoassay. Biosens Bioelectron 70:194–201
Gao Z, Hou L, Xu M, Tang D (2014) Enhanced colorimetric immunoassay accompanying with enzyme cascade amplification strategy for ultrasensitive detection of low-abundance protein. Sci Rep 4. doi:10.1038/srep03966
Que X, Tang D, Xia B, Lu M, Tang D (2014) Gold nanocatalyst-based immunosensing strategy accompanying catalytic reduction of 4-nitrophenol for sensitive monitoring of chloramphenicol residue. Anal Chim Acta 830:42–48
Zhan L, Li CM, Wu WB, Huang CZ (2014) A colorimetric immunoassay for respiratory syncytial virus detection based on gold nanoparticles-graphene oxide hybrids with mercury-enhanced peroxidase-like activity. Chem Commun 50(78):11526–11528
Mao X, Jiang J, Luo Y, Shen G, Yu R (2007) Copper-enhanced gold nanoparticle tags for electrochemical stripping detection of human IgG. Talanta 73(3):420–424
Kim D, Daniel WL, Mirkin CA (2009) Microarray-based multiplexed scanometric immunoassay for protein cancer markers using gold nanoparticle probes. Anal Chem 81(21):9183–9187
Liu Q, Jing C, Zheng X, Gu Z, Li D, Li D-W, Huang Q, Long Y-T, Fan C (2012) Nanoplasmonic detection of adenosine triphosphate by aptamer regulated self-catalytic growth of single gold nanoparticles. Chem Commun 48(77):9574–9576
Jana NR, Gearheart L, Murphy CJ (2001) Evidence for seed-mediated nucleation in the chemical reduction of gold salts to gold nanoparticles. Chem Mat 13(7):2313–2322
Long YJ, Li YF, Liu Y, Zheng JJ, Tang J, Huang CZ (2011) Visual observation of the mercury-stimulated peroxidase mimetic activity of gold nanoparticles. Chem Commun 47(43):11939–11941
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583
He W, Liu Y, Yuan J, Yin J-J, Wu X, Hu X, Zhang K, Liu J, Chen C, Ji Y, Guo Y (2011) Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials 32(4):1139–1147
Lee Y-C, Kim MI, Woo M-A, Park HG, Han J-I (2013) Effective peroxidase-like activity of a water-solubilized Fe-aminoclay for use inimmunoassay. Biosens Bioelectron 42:373–378
Gao Z, Xu M, Hou L, Chen G, Tang D (2013) Irregular-shaped platinum nanoparticles as peroxidase mimics for highly efficient colorimetric immunoassay. Anal Chim Acta 776:79–86
Wang Z, Yang X, Yang J, Jiang Y, He N (2015) Peroxidase-like activity of mesoporous silica encapsulated Pt nanoparticle and its application in colorimetric immunoassay. Anal Chim Acta 862:53–63
Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, Fernandez-Merino C, Vidal C (2007) Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 151:42–50
Tsai H, Lu Y-H, Liao H X, Wu S-W, Yu F Y, Fuh C (2015) Detection of rabbit IgG by using functional magnetic particles and an enzyme-conjugated antibody with a homemade magnetic microplate. Chem Cent J 9. doi:10.1186/s13065-015-0088-1
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (grant no. 21275158, 21575159), the 100 Talents Program of the Chinese Academy of Sciences, and the National Research Foundation of Korea (grant no. 2008-0061891 and 2009-00426).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1532 kb)
Rights and permissions
About this article
Cite this article
Wang, S., Chen, Z., Choo, J. et al. Naked-eye sensitive ELISA-like assay based on gold-enhanced peroxidase-like immunogold activity. Anal Bioanal Chem 408, 1015–1022 (2016). https://doi.org/10.1007/s00216-015-9219-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9219-8