Abstract
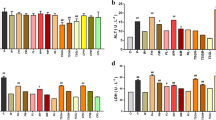
An in vitro cell metabolomics study was performed on human L02 liver cells to investigate the toxic biomarkers of pekinenal from the herb Euphorbia pekinensis Rupr. Pekinenal significantly induced L02 cell damage, which was characterised by necrosis and apoptosis. Metabolomics combined with data pattern recognition showed that pekinenal significantly altered the profiles of more than 1299 endogenous metabolites with variable importance in the projection (VIP) > 1. Further, screening correlation coefficients between the intensities of all metabolites and the extent of L02 cell damage (MTT) identified 12 biomarker hits: ten were downregulated and two were upregulated. Among these hits, LysoPC(18:1(9Z)/(11Z)), PC(22:0/15:0) and PC(20:1(11Z)/14:1(9Z)) were disordered, implying the initiation of inflammation and cell damage. Several fatty acids (FAs) (3-hydroxytetradecanedioic acid, pivaloylcarnitine and eicosapentaenoyl ethanolamide) decreased due to fatty acid oxidation. Dihydroceramide and Cer(d18:0/14:0) were also altered and are associated with apoptosis. Additional examination of the levels of intracellular reactive oxygen species (ROS) and two eicosanoids (PGE2, PGF2α) in the cell supernatant confirmed the fatty acid oxidation and arachidonic acid metabolism pathways, respectively. In summary, cell metabolomics is a highly efficient approach for identifying toxic biomarkers and helping understand toxicity mechanisms and predict herb-induced liver injury.

ᅟ






Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- BPI:
-
Base peak intensity
- CoA:
-
Coenzyme A
- COX:
-
Cyclooxygenase
- cPLA2:
-
Cytosolic phospholipase A2
- CS:
-
Ceramide synthase
- DCFH-DA:
-
2′,7′-Dichlorofluorescin diacetate
- DHCer:
-
Dihydroceramide
- DILI:
-
Drug-induced liver injury
- DMEM:
-
Dulbecco’s modified eagle medium
- DMSO:
-
Dimethyl sulfoxide
- EPEA:
-
Eicosapentaenoyl ethanolamide
- ER:
-
Endoplasmic reticulum
- ESI:
-
Electrospray ionisation
- FAs:
-
Fatty acids
- FBS:
-
Fetal bovine serum
- LPL:
-
Lipoprotein lipase
- LysoPCs:
-
Lysophosphatidylcholines
- MRM:
-
Multiple reaction monitoring
- OPLS-DA:
-
Orthogonal projection to latent structure discriminate analysis
- PCs:
-
Phosphatidylcholines
- PLS-DA:
-
Partial least squares discriminant analysis
- PPARα :
-
Peroxisome proliferator-activated receptor alpha
- ROS:
-
Reactive oxygen species
- S-plot:
-
Similarity plot
- UPLC-QTOF/MS:
-
Ultra-performance liquid chromatography-quadrupole time of flight/mass spectrometry
- UPLC-TQ/MS:
-
Ultra-performance liquid chromatography-triple quadrupole/mass spectrometry
- VIP:
-
Variable importance in the projection
References
Stickel F, Seitz HK (2000) The efficacy and safety of comfrey. Public Health Nutr 3:501–508. doi:10.1017/S1368980000000586
Kaplowitz N, DeLeve LD (2013) Drug-induced liver disease. Elsevier, Amsterdam
Jung KA, Min HJ, Yoo SS, Kim HJ, Choi SN, Ha CY, Kim HJ, Kim TH, Jung WT, Lee OJ, Lee JS, Shim SG (2011) Drug-induced liver injury: twenty five cases of acute hepatitis following ingestion of polygonum multiflorum Thunb. Gut Liver 5:493–499. doi:10.5009/gnl.2011.5.4.493
Huang WQ, Luo Y (2004) Influence of licorice root and Peking euphorbia root in combination on functions and pathological morphology of the heart, liver and kidney in rats. Chin J Clin Rehabil 8:6804–6805
O’Connell TM, Watkins PB (2010) The application of metabonomics to predict drug-induced liver injury. Clin Pharmacol Ther 88:394–399. doi:10.1038/clpt.2010.151
Nicholson JK, Wilson ID (2003) Opinion: understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov 2:668–676. doi:10.1038/nrd1157
Salek RM, Maguire ML, Bentley E, Rubtsov DV, Hough T, Cheeseman M, Nunez D, Sweatman BC, Haselden JN, Cox RD, Connor SC, Griffin JL (2007) A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol Genomics 29:99–108. doi:10.1152/physiolgenomics.00194.2006
Tao W, Duan J, Tang Y, Yang N, Li J, Qian Y (2013) Casbane diterpenoids from the roots of euphorbia pekinensis. Phytochemistry 94:249–253. doi:10.1016/j.phytochem.2013.06.009
Su S, Duan J, Wang P, Liu P, Guo J, Shang E, Qian D, Tang Y, Tang Z (2013) Metabolomic study of biochemical changes in the plasma and urine of primary dysmenorrhea patients using UPLC-MS coupled with a pattern recognition approach. J Proteome Res 12:852–865. doi:10.1021/pr300935x
Martano G, Delmotte N, Kiefer P, Christen P, Kentner D, Bumann D, Vorholt JA (2015) Fast sampling method for mammalian cell metabolic analyses using liquid chromatography-mass spectrometry. Nat Protoc 10:1–11. doi:10.1038/nprot.2014.198
Hsu F, Turk J (1999) Structural characterization of triacylglycerols as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisionally activated dissociation on a triple stage quadrupole instrument. J Am Soc Mass Spectrom 10:587–600. doi:10.1016/S1044-0305(99)00035-5
Liu-Wu Y, Hurt-Camejo E, Wiklund O (1998) Lysophosphatidylcholine induces the production of IL-1beta by human monocytes. Atherosclerosis 137:351–357. doi:10.1016/S0021-9150(97)00295-5
Matsumoto M, Kuhara T, Inoue Y, Shinka T, Matsumoto I (1991) Abnormal fatty acid metabolism in patients in hopantenate therapy during clinical episodes. J Chromatog B 562:139–145. doi:10.1016/0378-4347(91)80572-T
Chen K, Cheng M, Jing Y, Chiu DT, Shiao M, Chen J (2011) Resveratrol ameliorates metabolic disorders and muscle wasting in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab 301:E853–E863. doi:10.1152/ajpendo.00048.2011
Brass EP (2002) Pivalate-generating prodrugs and carnitine homeostasis in man. Pharmacol Rev 54:589–598. doi:10.1124/pr.54.4.589
Nakajima Y, Ito T, Maeda Y, Ichiki S, Sugiyama N, Mizuno M, Makino Y, Sugiura T, Kurono Y, Togari H (2010) Detection of pivaloylcarnitine in pediatric patients with hypocarnitinemia after long-term administration of pivalate-containing antibiotics. Tohoku J Exp Med 221:309–313. doi:10.1620/tjem.221.309
Stiban J, Fistere D, Colombini M (2006) Dihydroceramide hinders ceramide channel formation: implications on apoptosis. Apoptosis 11:773–780. doi:10.1007/s10495-006-5882-8
Soriano JM, González L, Catalá AI (2005) Mechanism of action of sphingolipids and their metabolites in the toxicity of fumonisin B. Prog Lipid Res 44:345–356. doi:10.1016/j.plipres.2005.09.001
Hu W, Xu R, Zhang G, Jin J, Szulc ZM, Bielawski J, Hannun YA, Obeid LM, Mao C (2005) Golgi fragmentation is associated with ceramide-induced cellular effects. Mol Biol Cell 16:1555–1567. doi:10.1091/mbc.E04-07-0594
Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G (2006) Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17:571–588. doi:10.1016/j.jnutbio.2005.12.001
Yao W, Gu H, Zhu J, Barding G, Cheng H, Bao B, Zhang L, Ding A, Li W (2014) Integrated plasma and urine metabolomics coupled with HPLC/QTOF-MS and chemometric analysis on potential biomarkers in liver injury and hepatoprotective effects of Er-Zhi-Wan. Anal Bioanal Chem 406:7367–7378. doi:10.1007/s00216-014-8169-x
Bohgaki M, Tsukiyama T, Nakajima A, Maruyama S, Watanabe M, Koike T, Hatakeyama S (2008) Involvement of Ymer in suppression of NF-κB activation by regulated interaction with lysine-63-linked polyubiquitin chain. Biochim Biophys Acta 1783:826–837. doi:10.1016/j.bbamcr.2007.09.006
Miura D, Tanaka H, Wariishi H (2004) Metabolomic differential display analysis of the white-rot basidiomycete Phanerochaete chrysosporium grown under air and 100% oxygen. FEMS Microbiol Lett 234:111–116. doi:10.1111/j.1574-6968.2004.tb09521.x
Madsen RK, Lundstedt T, Gabrielsson J, Sennbro C, Alenius G, Moritz T, Rantapää-Dahlqvist S, Trygg J (2011) Diagnostic properties of metabolic perturbations in rheumatoid arthritis. Arthritis Res Ther 13:R19. doi:10.1186/ar3243
Mattes W, Davis K, Fabian E, Greenhaw J, Herold M, Looser R, Mellert W, Groeters S, Marxfeld H, Moeller N, Montoya-Parra G, Prokoudine A, van Ravenzwaay B, Strauss V, Walk T, Kamp H (2014) Detection of hepatotoxicity potential with metabolite profiling (metabolomics) of rat plasma. Toxicol Lett 230:467–478. doi:10.1016/j.toxlet.2014.07.021
Wang J, Pu S, Sun Y, Li Z, Niu M, Yan X, Zhao Y, Wang L, Qin X, Ma Z, Zhang Y, Li B, Luo S, Gong M, Sun Y, Zou Z, Xiao X (2014) Metabolomic profiling of autoimmune hepatitis: the diagnostic utility of nuclear magnetic resonance spectroscopy. J Proteome Res 13:3792–3801. doi:10.1021/pr500462f
Acknowledgments
The project is supported by National Basic Research Program of China (973 Program) (2011CB505300, 2011CB505303), National Natural Science Foundation of China (81274199, 81102762, 30901894 and 81403041), Open Fund of Collaborative Innovation Center of Chinese Medicinal Resources Industrialization (ZDXM-1-14), Six talent peaks project in Jiangsu Province (YY-015),the Natural Science Foundation of Jiangsu Province (BK20140961) and a project founded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest, either real or potential, associated with this work.
Additional information
Jiexia Shi and Jing Zhou contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 152 kb)
Rights and permissions
About this article
Cite this article
Shi, J., Zhou, J., Ma, H. et al. An in vitro metabolomics approach to identify hepatotoxicity biomarkers in human L02 liver cells treated with pekinenal, a natural compound. Anal Bioanal Chem 408, 1413–1424 (2016). https://doi.org/10.1007/s00216-015-9202-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9202-4