Abstract
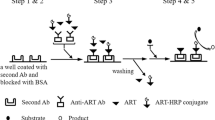
Despite significant progress in prevention and therapy, malaria is still one of the world’s leading major diseases due to its high morbidity and mortality. Recommended treatments by the World Health Organization include the use of artemisinin and artemisinin derivative-based combination therapies. To allow efficient patient monitoring during antimalarial therapy without the use of expensive apparatus, we developed a sensitive direct chemiluminescent enzyme immunoassay for the determination of dihydroartemisinin in biological fluids. To produce specific antibodies against dihydroartemisinin (DHA), a synthetic DHA derivative was coupled to bovine serum albumin as the immunogen. In parallel, a new, rapid, and efficient procedure to covalently link glycoprotein to all amine-containing molecules has been established and the enzyme tracer was prepared by chemically coupling the DHA derivative in combination with SBP rather than the more commonly used HRP. It allowed us to develop, after optimization of the luminescent reagent, a sensitive and stable luminescent EIA, with a LLOQ of 90 pg mL−1. This assay compares favorably with the most efficient HPLC methods previously reported with a LLOQ close to 1 ng mL−1 and shows good precision and efficiency since recovery from human plasma spiked with DHA ranged between 91 and 103 %, with coefficients of variation of <13 %. To date, no immunoassay for DHA has been applied to plasma analysis and this EIA should be very useful in all clinical laboratories for rapid and cost-effective analysis.

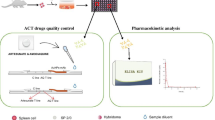
DHA chemical structure and chemiluminescent immunoassay standard curve



Similar content being viewed by others
References
White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM (2014) Lancet 383:723–735
German PI, Aweeka FT (2008) Clin Pharmacokinet 47:91–102
Klein EY (2013) Int J Antimicrob Agents 41:311–317
Gu Y, Li Q, Melendez V, Weina P (2008) J Chromatogr B Anal Technol Biomed Life Sci 867:213–218
Hilhorst MJ, Hendriks G, de Vries R, Hillewaert V, Verhaege T, van de Merbel NC (2014) J Chromatogr B Anal Technol Biomed Life Sci 965:45–53
Huang L, Jayewardene AL, Li X, Marzan F, Lizak PS, Aweeka FT (2009) J Pharm Biomed Anal 50:959–965
Van Quekelberghe SA, Soomro SA, Cordonnier JA, Jansen FH (2008) J Anal Toxicol 32:133–139
Hodel EM, Zanolari B, Mercier T, Biollaz J, Keiser J, Olliaro P, Genton B, Decosterd LA (2009) J Chromatogr B Anal Technol Biomed Life Sci 877:867–886
Lai CS, Nair NK, Muniandy A, Mansor SM, Olliaro PL, Navaratnam V (2009) J Chromatogr B Anal Technol Biomed Life Sci 877:558–562
Ferreira JF, Janick J (1996) Phytochemistry 41:97–104
He SP, Tan GY, Li G, Tan WM, Nan TG, Wang BM, Li ZH, Li QX (2009) Anal Bioanal Chem 393:1297–1303
Tanaka H, Putalun W, De-Eknamkul W, Matangkasombut O, Shoyama Y (2007) Planta Med 73:1127–1132
He L, Nan T, Cui Y, Guo S, Zhang W, Zhang R, Tan G, Wang B, Cui L (2014) Malar J 13:127
Henriksen A, Mirza O, Indiani C, Teilum K, Smulevich G, Welinder KG, Gajhede M (2001) Protein Sci 10:108–115
Marzocchi E, Grilli S, Della CL, Prodi L, Mirasoli M, Roda A (2008) Anal Biochem 377:189–194
Azoulay S, Nevers MC, Creminon C, Heripret L, Durant J, Dellamonica P, Grassi J, Guedj R, Duval D (2004) Antimicrob Agents Chemother 48:104–109
Roucairol C, Azoulay S, Nevers MC, Creminon C, Grassi J, Burger A, Duval D (2007) Anal Chim Acta 589:142–149
D’Acquarica I, Gasparrini F, Kotoni D, Pierini M, Villani C, Cabri W, Di MM, Giorgi F (2010) Molecules 15:1309–1323
Singh C, Chaudhary S, Puri SK (2006) J Med Chem 49:7227–7233
Roda A, Guardigli M (2012) Anal Bioanal Chem 402:69–76
Girotti S, Ghini S, Maiolini E, Bolelli L, Ferri EN (2013) Anal Bioanal Chem 405:555–571
Ryan BJ, Carolan N, O’Fagain C (2006) Trends Biotechnol 24:355–363
Sakharov IY, Alpeeva IS, Efremov EE (2006) J Agric Food Chem 54:1584–1587
Sakharov IY, Berlina AN, Zherdev AV, Dzantiev BB (2010) J Agric Food Chem 58:3284–3289
Tijssen P, Kurstak E (1984) Anal Biochem 136:451–457
Vdovenko MM, Vorob’ev AK, Sakharov II (2013) Bioorg Khim 39:200–205
Yu FY, Vdovenko MM, Wang JJ, Sakharov IY (2011) J Agric Food Chem 59:809–813
Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin CS, Fleckenstein L (2011) Malar J 10:263
Akpaloo W, Purssell E (2014) Malar Res Treat 2014:263674
Naing C, Racloz V, Whittaker MA, Aung K, Reid SA, Mak JW, Tanner M (2013) PLoS One 8:e78819
Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Sugiarto P, Tjitra E, Anstey NM, Price RN (2015) Malar J 14:272
Acknowledgments
This work was supported in part by Fond Unique Interministeriel (6th call – FUI) and Caisse d’Assurance Maladie des Professions Liberales.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zehnacker, L., Nevers, MC., Sinou, V. et al. Development of sensitive direct chemiluminescent enzyme immunoassay for the determination of dihydroartemisinin in plasma. Anal Bioanal Chem 407, 7823–7830 (2015). https://doi.org/10.1007/s00216-015-8951-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8951-4