Abstract
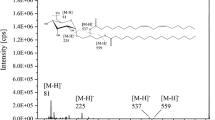
Among the goals of lipidomics applied to triacylglycerols (TAGs) is identification of molecular species, degree and location of unsaturation, and positions of fatty acyl chains (i.e., identification of regioisomers). Toward those ends, we define one, two, and three “Critical Ratios” for Types I, II, and III TAGs that provided different aspects of the desired information. Critical Ratio 1, [MH]+/Σ[DAG]+, is correlated to the degree of unsaturation ([MH]+ is the protonated molecule and Σ[DAG]+ is the sum of diacylglycerol-like ions, [DAG]+); Critical Ratio 2, [AA]+/[AB]+ for Type II TAGs (“ABA/AAB/BAA”) and [AC]+/([AB]++[BC]+) for Type III TAGs (“ABC/CBA/BAC/CAB/ACB/BCA”), is correlated to identification of regioisomers; and Critical Ratio 3, [BC]+/[AB]+, provides information about those [DAG]+ from Type III TAGs. Furthermore, Critical Ratios are used in the Updated Bottom Up Solution (UBUS) to reproduce the mass spectra of TAGs by atmospheric pressure chemical ionization mass spectrometry applied to analysis of soybean oil in a dietary supplement gelcap. We present a new model for the [MH]+/Σ[DAG]+ ratio, quantify regioisomers using the [AA]+/[AB]+ ratio, and describe trends for [BC]+/[AB]+ that have never been reported before. The UBUS is also applied to other classes of molecules, i.e., vitamin D and DAGs. The amount of vitamin D3 in the gelcap fell from 2011 ± 22 when received to 1689 ± 33 just prior to expiration. The Critical Ratios constitute a compact data set that can provide structural information and also act as a library of mass spectra.

The shape of the Updated Bottom Up Solution for Type II triacylglycerols








Similar content being viewed by others
References
Hunter JE (2001) Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 36(7):655–668
Schwab U, Lauritzen L, Tholstrup T, Haldorssoni T, Riserus U, Uusitupa M, Becker W (2014) Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutri Res 58. doi:10.3402/fnr.v58.25145
Bézard J, Blond JP, Bernard A, Clouet P (1994) The metabolism and availability of essential fatty acids in animal and human tissues. Reprod Nutr Dev 34(6):539–568
Mu H, Porsgaard T (2005) The metabolism of structured triacylglycerols. Prog Lipid Res 44(6):430–448. doi:10.1016/j.plipres.2005.09.002
Karupaiah T, Sundram K (2007) Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: a review of their nutritional implications. Nutr Metab 4. doi:10.1186/1743-7075-4-16
Dubois V, Breton S, Linder M, Fanni J, Parmentier M (2007) Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Technol 109(7):710–732. doi:10.1002/ejlt.200700040
Byrdwell WC, Emken EA (1995) Analysis of triglycerides using atmospheric pressure chemical ionization mass spectrometry. Lipids 30(2):173–175. doi:10.1007/BF02538272
Neff WE, Byrdwell WC (1995) Soybean oil triacylglycerol analysis by reversed-phase high-performance liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry. JAOCS, J Am Oil Chem Soc 72(10):1185–1191
Byrdwell WC, Emken EA, Neff WE, Adlof RO (1996) Quantitative analysis of triglycerides using atmospheric pressure chemical ionization-mass spectrometry. Lipids 31(9):919–935
Laakso P, Voutilainen P (1996) Analysis of triacylglycerols by silver-ion high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. Lipids 31(12):1311–1322
Laakso P (1997) Characterization of alpha- and gamma-linolenic acid oils by reversed-phase high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. JAOCS, J Am Oil Chem Soc 74(10):1291–1300
Duffin KL, Henion JD, Shieh JJ (1991) Electrospray and tandem mass spectrometric characterization of acylglycerol mixtures that are dissolved in nonpolar solvents. Anal Chem 63(17):1781–1788
Cheng C, Gross ML, Pittenauer E (1998) Complete structural elucidation of triacylglycerols by tandem sector mass spectrometry. Anal Chem 70(20):4417–4426
Schuyl PJW, De Joode T, Vasconcellos MA, Duchateau GSMJE (1998) Silver-phase high-performance liquid chromatography-electrospray mass spectrometry of triacylglycerols. J Chromatogr A 810(1–2):53–61. doi:10.1016/S0021-9673(98)00277-5
Hsu FF, Turk J (1999) Structural characterization of triacylglycerols as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisionally activated dissociation on a triple stage quadrupole instrument. J Am Soc Mass Spectrom 10(7):587–599
Hvattum E (2001) Analysis of triacylglycerols with non-aqueous reversed-phase liquid chromatography and positive ion electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom 15(3):187–190. doi:10.1002/1097-0231(20010215)15:3<187::AID-RCM211>3.0.CO;2-T
Marzilli LA, Fay LB, Dionisi F, Vouros P (2003) Structural characterization of triacylglycerols using electrospray ionization-MSn ion-trap MS. JAOCS, J Am Oil Chem Soc 80(3):195–202
Byrdwell WC (2001) Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids. Lipids 36(4):327–346
Mottram HR (2005) Regioisomeric analysis of triacylglycerols using high performance liquid chromatography/ atmospheric pressure chemical ionization mass spectrometry. In: Byrdwell WC (ed) Modern Methods for Lipid Analysis by Liquid Chromatography/ Mass Spectrometry and Related Techniques. AOCS Press, Champaign, pp 276–297
Řezanka T, Sigler K (2007) The use of atmospheric pressure chemical ionization mass spectrometry with high performance liquid chromatography and other separation techniques for identification of triacylglycerols. Curr Anal Chem 3(4):252–271. doi:10.2174/157341107782109644
Byrdwell WC (2005) Qualitative and quantitative analysis of triacylglycerols by atmospheric pressure ionization (APCI and ESI) mass spectrometry techniques. In: Byrdwell WC (ed) Modern methods for lipid analysis by liquid chromatography/ mass spectrometry and related techniques. AOCS Press, Champaign, pp 298–412
Murphy RC, Leiker TJ, Barkley RM (2011) Glycerolipid and cholesterol ester analyses in biological samples by mass spectrometry. Biochim Biophys Acta Mol Cell Biol Lipids 1811(11):776–783. doi:10.1016/j.bbalip.2011.06.019
Cozzolino R, De Giulio B (2011) Application of ESI and MALDI-TOF MS for triacylglycerols analysis in edible oils. Eur J Lipid Sci Technol 113(2):160–167
Sandra K, Sandra P (2013) Lipidomics from an analytical perspective. Curr Opin Chem Biol 17(5):847–853. doi:10.1016/j.cbpa.2013.06.010
Cajka T, Fiehn O (2014) Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. TrAC Trends Anal Chem 61:192–206. doi:10.1016/j.trac.2014.04.017
Mottram HR, Evershed RP (1996) Structure analysis of triacylglycerol positional isomers using atmospheric pressure chemical ionisation mass spectrometry. Tetrahedron Lett 37(47):8593–8596
Fauconnot L, Hau J, Aeschlimann JM, Fay LB, Dionisi F (2004) Quantitative analysis of triacylglycerol regioisomers in fats and oils using reversed-phase high-performance liquid chromatography and atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom 18(2):218–224
Byrdwell WC (2005) The bottom-up solution to the triacylglycerol lipidome using atmospheric pressure chemical ionization mass spectrometry. Lipids 40(4):383–417. doi:10.1007/s11745-006-1398-9
Holcapek M, Dvorakova H, Lisa M, Giron AJ, Sandra P, Cvacka J (2010) Regioisomeric analysis of triacylglycerols using silver-ion liquid chromatography-atmospheric pressure chemical ionization mass spectrometry: comparison of five different mass analyzers. J Chromatogr A 1217(52):8186–8194
Jakab A, Jablonkai I, Forgács E (2003) Quantification of the ratio of positional isomer dilinoleoyl-oleoyl glycerols in vegetable oils. Rapid Commun Mass Spectrom 17(20):2295–2302
Manninen P, Laakso P (1997) Capillary supercritical fluid chromatography-atmospheric pressure chemical ionization mass spectrometry of triacylglycerols in berry oils. JAOCS, J Am Oil Chem Soc 74(9):1089–1098
Byrdwell WC (2011) “Dilute-and-shoot” triple parallel mass spectrometry method for analysis of vitamin D and triacylglycerols in dietary supplements. Anal Bioanal Chem 401(10):3317–3334. doi:10.1007/s00216-011-5406-4
Byrdwell WC (2013) Quadruple parallel mass spectrometry for analysis of vitamin D and triacylglycerols in a dietary supplement. J Chromatogr A 1320:48–65. doi:10.1016/j.chroma.2013.10.031
Byrdwell WC, Neff WE, List GR (2001) Triacylglycerol analysis of potential margarine base stocks by high-performance liquid chromatography with atmospheric pressure chemical ionization mass spectrometry and, flame ionization detection. J Agric Food Chem 49(1):446–457. doi:10.1021/jf0008801
Byrdwell WC (2014) Extract-filter-shoot liquid chromatography with mass spectrometry for the analysis of vitamin D2 in a powdered supplement capsule and standard reference material 3280. J Sep Sci 37(16):2095–2110. doi:10.1002/jssc.201400234
Byrdwell WC, Neff WE (1997) Qualitative and quantitative analysis of triacylglycerols using atmospheric pressure chemical ionization mass spectrometry. In: McDonald RE, Mossoba MM (eds) New techniques and applications in lipid analysis. AOCS Press, Champaign, pp 45–80
Yamamoto H (1977) Calculations of isotopic distribution in molecules extensively labeled with heavy isotopes. Anal Chem 49(2):281–283
Patiny L, Borel A (2013) ChemCalc: a building block for tomorrow’s chemical infrastructure. J Chem Inf Model 53(5):1223–1228. doi:10.1021/ci300563h
Acknowledgments
The work of Dr. Robert Goldschmidt in assisting with some integration and reporting functions is gratefully acknowledged. This work was supported by the USDA Agricultural Research Service. Mention or use of specific products or brands do not represent or imply endorsement by the USDA.
Compliance with ethical standards
The author declares no potential conflicts of interest with respect to the performance of experiments, authorship, or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Lipidomics with guest editor Michal Holčapek.
Rights and permissions
About this article
Cite this article
Byrdwell, W.C. The Updated Bottom Up Solution applied to mass spectrometry of soybean oil in a dietary supplement gelcap. Anal Bioanal Chem 407, 5143–5160 (2015). https://doi.org/10.1007/s00216-015-8590-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8590-9