Abstract
Doped organically modified silica nanoparticles (ORMOSIL NPs) with luminescent molecules represent a potent approach to signal amplification in biomolecule labeling. Herein, we report the synthesis of new ORMOSIL NPs incorporating thermochemiluminescent (TCL) 1,2-dioxetane derivatives to prepare TCL labels for ultrasensitive immunoassay, displaying a detectability comparable to those offered by other conventional luminescence-based systems. Amino-functionalized ORMOSIL NPs were synthesized for inclusion of acridine-containing 1,2-dioxetane derivatives with a fluorescence energy acceptor. The doped ORMOSIL NPs were further functionalized with biotin for binding to streptavidin-labeled species to be used as universal detection reagents for immunoassays. A quantitative non-competitive immunoassay for streptavidin has been developed by immobilizing anti-streptavidin antibody to capture streptavidin, then the antibody-bound streptavidin was detected by the biotinylated TCL ORMOSIL NPs. The analytical performance was similar to that obtained by chemiluminescent (CL) detection using horseradish peroxidase (HRP) as label, being the limits of detection 2.5–3.8 and 0.8 ng mL−1 for TCL and CL detection, respectively. In addition, since the TCL emission is simply initiated by thermolysis of the label, chemical reagents were not required, thus allowing reagentless detection with a simplification of the analytical protocols. A compact mini dark box device based on the use of a cooled charge-coupled device (CCD) and a miniaturized heater has been developed and used to quantify the light emission after heat decomposition of the label at a temperature of 90–120 °C. These characteristics make TCL-doped ORMOSIL NPs ideal universal nanoprobes for ultrasensitive bioassays such as immuno- and DNA-based assay.

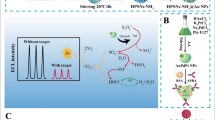
Schematic representation of the silica nanoparticles decorated with biotin and containing 1,2-dioxetane derivatives and the fluorescent energy acceptor BPEA (left); thermochemiluminescence images obtained for a model streptavidin immunoassay (right)






Similar content being viewed by others
References
Roda A, Guardigli M (2012) Analytical chemiluminescence and bioluminescence: latest achievements and new horizons. Anal Bioanal Chem 402:69–76
Richter MM (2004) Electrochemiluminescence (ECL). Chem Rev 104:3003–3036
Mirasoli M, Guardigli M, Michelini E, Roda A (2014) Recent advancements in chemical luminescence-based lab-on-chip and microfluidic platforms for bioanalysis. J Pharm Biomed Anal 87:36–52
Luider TM, Hummelen JC, Koek JN, Oudman D, Wynberg H (1990) In: van Dyke K, van Dyke R (eds) Luminescence immunoassay and molecular applications. CRC Press, Boca Raton
Hummelen JC, Luider TM, Wynberg H (1986) Stable 1,2-dioxetanes as labels for thermochemiluminescent immunoassay. Methods Enzymol 133:531–557
Hummelen JC, Luider TM, Wynberg H (1988) In: Collins WP (ed) Complementary immunoassays. John Wiley & Sons, Chichester
Roda A, Di Fusco M, Quintavalla A, Guardigli M, Mirasoli M, Lombardo M, Trombini C (2012) Dioxetane-doped silica nanoparticles as ultrasensitive reagentless thermochemiluminescent labels for bioanalytics. Anal Chem 84:9913–9919
Di Fusco M, Quintavalla A, Trombini C, Lombardo M, Roda A, Guardigli M, Mirasoli M (2013) Preparation and characterization of thermochemiluminescent acridine-containing 1,2-dioxetanes as promising ultrasensitive labels in bioanalysis. J Org Chem 78:11238–11246
Miralles V, Huerre A, Malloggi F, Jullien M-C (2013) A review of heating and temperature control in microfluidic systems: techniques and applications. Diagnostics 3:33–67
Wu J, Kodzius R, Cao W, Wen W (2013) Extraction, amplification and detection of DNA in microfluidic chip-based assays. Microchim Acta 181:1611–1631
Marzocchi E, Grilli S, Della Ciana L, Prodi L, Mirasoli M, Roda A (2008) Chemiluminescent detection systems of horseradish peroxidase employing nucleophilic acylation catalysts. Anal Biochem 377:189–194
Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, Dougherty TJ, Prasad PN (2003) Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: a novel drug-carrier system for photodynamic therapy. J Am Chem Soc 125:7860–7865
Jain TK, Roy I, De TK, Maitra AN (1998) Nanometer silica particles encapsulating active compounds: a novel ceramic drug carrier. J Am Chem Soc 120:11092–11095
Bonacchi S, Genovese D, Juris R, Montalti M, Prodi L, Rampazzo E, Zaccheroni N (2011) Luminescent silica nanoparticles: extending the frontiers of brightness. Angew Chem Int Ed 50:4056–4066
Roy I, Ohulchanskyy TY, Bharali DJ, Pudavar HE, Mistretta RA, Kaur N, Prasad PN (2005) Optical tracking of organically modified silica nanoparticles as DNA carriers: a nonviral, nanomedicine approach for gene delivery. Proc Natl Acad Sci USA 102:279–284
Hermanson GT (1996) Bioconjugate techniques, 2nd edn. Academic, San Diego
Li D, Frey MW, Vynias D, Baeumner AJ (2007) Availability of biotin incorporated in electrospun PLA fibers for streptavidin binding. Polymer 48:6340–6347
Roda A, Mirasoli M, Dolci LS, Buragina A, Bonvicini F, Simoni P, Guardigli M (2011) Portable device based on chemiluminescence lensless imaging for personalized diagnostics through multiplex bioanalysis. Anal Chem 83:3178–3185
Mirasoli M, Buragina A, Dolci LS, Guardigli M, Simoni P, Montoya A, Maiolini E, Girotti S, Roda A (2012) Development of a chemiluminescence-based quantitative lateral flow immunoassay for on-field detection of 2,4,6-trinitrotoluene. Anal Chim Acta 721:167–172
Mirasoli M, Bonvicini F, Dolci LS, Zangheri M, Gallinella G, Roda A (2013) Portable chemiluminescence multiplex biosensor for quantitative detection of three B19 DNA genotypes. Anal Bioanal Chem 405:1139–1143
Lee DC-S, Wilson T (1973) In: Cormier MJ, Hercules M, Lee J (eds) Chemiluminescence and bioluminescence. New York, Plenum
Adam W, Heil M (1992) Reaction of 1,2-dioxetanes with heteroatom nucleophiles: adduct formation by nucleophilic attack at the peroxide bond. J Am Chem Soc 114:5591–5598
Rao MS, Gray J, Dave BC (2003) Smart glasses: molecular programming of dynamic responses in organosilica sol-gels. J Sol-Gel Sci Technol 26:553–560
Atluri R, Sakamoto Y, Garcia-Bennett AE (2009) Co-structure directing agent induced phase transformation of mesoporous materials. Langmuir 25:3189–3195
Berlman IB (1971) Handbook of fluorescence spectra of aromatic molecules, 1st edn. Academic, New York
Borisevitcha IE, Tabak M (1992) Electronic absorption and fluorescence spectroscopic studies of dipyridamole: effects of solution composition. J Lumin 51:315–322
Zaklika KA, Kissel T, Thayer AL, Burns PA, Schaap AP (1979) Mechanism of 1,2-dioxetane decomposition: the role of electron transfer. Photochem Photobiol 30:35–40
Menger FM, Jerkunica JM, Johnston JC (1978) The water content of a micelle interior. The Fjord vs. Reef models. J Am Chem Soc 100:4676–4678
Casal HL (1988) On the water content of micelles: infrared spectroscopic studies. J Am Chem Soc 110:5203–5205
Kuhn H, Breitzke B, Rehage H (1998) The phenomenon of water penetration into sodium octanoate micelles studied by molecular dynamics computer simulation. Colloid Polym Sci 276:824–832
Zaklika KA, Burns PA, Schaap AP (1978) Enhanced chemiluminescence from the silica gel catalyzed decomposition of a 1,2-dioxetane. J Am Chem Soc 100:318–320
Mirasoli M, Nascetti A, Caputo D, Zangheri M, Scipinotti R, Cevenini L, de Cesare G, Roda A (2014) Multiwell cartridge with integrated array of amorphous silicon photosensors for chemiluminescence detection: development, characterization and comparison with cooled-CCD luminograph. Anal Bioanal Chem 406:5645–5656
Acknowledgments
This work was supported by the University of Bologna (FARB Programme), MIUR, and the 2007–2013 Emilia Romagna Regional Operational Programme (ROP) of the European Regional Development Fund (ERDF).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 428 kb)
Rights and permissions
About this article
Cite this article
Di Fusco, M., Quintavalla, A., Lombardo, M. et al. Organically modified silica nanoparticles doped with new acridine-1,2-dioxetane analogues as thermochemiluminescence reagentless labels for ultrasensitive immunoassays. Anal Bioanal Chem 407, 1567–1576 (2015). https://doi.org/10.1007/s00216-014-8406-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8406-3