Abstract
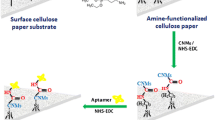
A combination of thin-film microextaction based on an aptamer immobilized on modified Whatman cellulose paper followed by electrospray ionization ion mobility spectrometry has been developed for the analysis of codeine in urine samples. The immobilization is based on the covalent linking of an amino-modified anticodeine aptamer to aldehyde groups of the oxidized cellulose paper. The covalent bonds were examined by infrared spectroscopy and elemental analysis. The effect of the extraction parameters, including the elution conditions (solvent type and volume), extraction time, and extraction temperature, on the extraction efficiency were investigated. Under the optimized conditions, the linear dynamic range was found to be 10-300 ng/mL with a detection limit of 3.4 ng/mL for codeine in urine. The relative standard deviation was 6.8 % for three replicate measurements of codeine at 100 ng/mL in urine. Furthermore, the samples were analyzed with a standard method for the analysis of codeine using high-performance liquid chromatography with ultraviolet detection. The comparison of the results validates the accuracy of the proposed method as an alternative method for the analysis of codeine in urine samples.

A combination of TFME based on aptamer immobilized on modified Whatman cellulose paper with ESIIMS has been developed. This method was used for the determination of codeine in urine samples





Similar content being viewed by others
References
Scorrano S, Longo L, Vasapollo G (2010) Molecularly imprinted polymers for solid-phase extraction of 1-methyladenosine from human urine. Anal Chim Acta 659:167–171
Mascini M (2008) Analytical applications of aptamers. Hacet J Biol Chem 36:273–282
Balamurugan S, Obubuafo A, Soper SA, Spivak DA (2008) Surface immobilization methods for aptamer diagnostic applications. Anal Bioanal Chem 390:1009–1021
Stoltenburg R, Reinemann C, Strehlitz B (2005) FluMag-SELEX as an advantageous method for DNA aptamer selection. Anal Bioanal Chem 383:83–91
White R, Rusconi C, Scardino E, Wolberg A, Lawson J, Hoffman M, Sullenger B (2001) Generation of species cross-reactive aptamers using “toggle” SELEX. Mol Ther 4:567–574
Ravelet C, Grosst C, Peyrin E (2006) Liquid chromatography, electrochromatography and capillary electrophoresis applications of DNA and RNA aptamers. J Chromatogr A 1117:1–10
Deng Q, Watson CJ, Kennedy RT (2003) Aptamer affinity chromatography for rapid assay of adenosine in microdialysis samples collected in vivo. J Chromatogr A 1005:123–130
Najafi Aslipashaki S, Khayamian T, Hashemian Z (2013) Aptamer based extraction followed by electrospray ionization-ion mobility spectrometry for analysis of tetracycline in biological fluids. J Chromatogr B 925:26–32
Centi S, Tombelli S, Minunni M, Mascini M (2007) Aptamer-based detection of plasma proteins by an electrochemical assay coupled to magnetic beads. Anal Chem 79:1466–1473
Wu X, Hu J, Zhu B, Lu L, Huang X, Pang D (2011) Aptamer-targeted magnetic nanospheres as a solid-phase extraction sorbent for determination of ochratoxin A in food samples. J Chromatogr A 1218:7341–7346
Liu W, Wei H, Lin Z, Mao S, Lin JM (2011) Rare cell chemiluminescence detection based on aptamer-specific capture in microfluidic channels. Biosens Bioelectron 28:438–442
Mu L, Hu X, Wen J, Zhou Q (2013) Robust aptamer sol–gel solid phase microextraction of very polar adenosine from human plasma. J Chromatogr A 1279:7–12
Du F, Alam MN, Pawliszyn J (2014) Aptamer-functionalized solid phase microextraction–liquid chromatography/tandem mass spectrometry for selective enrichment and determination of thrombin. Anal Chim Acta 845:45–52
Jiang R, Pawliszyn J (2012) Thin-film microextraction offers another geometry for solid-phase microextraction. Trends Anal Chem 39:245–253
Saraji M, Farajmand B (2013) Chemically modified cellulose paper as a thin filmmicroextraction phase. J Chromatogr A 1314:24–30
Diankova SM, Doneva MD (2009) Analysis of oxycellulose obtained by partial oxidation with different reagents. Bulg Chem Commun 41:391–396
Ochoa ML, Harrington PB (2004) Detection of methamphetamine in the presence of nicotine using in situ chemical derivatization and ion mobility spectrometry. Anal Chem 76:985–992
Huang L, Yang X, Qi C, Niu X, Zhao C, Zhao X, Shangguan D, Yang Y (2013) A label-free electrochemical biosensor based on a DNA aptamer against codeine. Anal Chim Acta 787:203–210
Arabzadeh N, Khayamian T (2012) Pneumatically assisted electrospray-ion mobility spectrometry for quantitative analysis of intact proteins. Talanta 99:29–35
Khayamian T, Jafari MT (2007) Design for electrospray ionization-ion mobility spectrometry. Anal Chem 79:3199–3205
Su S, Nutiu R, Filipe CDM, Li Y, Pelton R (2007) Adsorption and covalent coupling of ATP-binding DNA aptamers onto cellulose. Langmuir 23:1300–1302
Karpas Z (1989) Ion mobility spectrometry of aliphatic and aromatic amines. Anal Chem 61:684–689
Matz LM, Hill HH Jr (2001) Evaluation of opiate separation by high-resolution electrospray ionization-ion mobility spectrometry/mass spectrometry. Anal Chem 73:1664–1669
Sarafraz-Yazdi A, Amiri A, Rounaghi G, Eshtiagh-Hosseini H (2012) Determination of non-steroidal anti-inflammatory drugs in water samples by solid-phase microextraction based sol–gel technique using poly(ethylene glycol) grafted multi-walled carbon nanotubes coated fiber. Anal Chim Acta 720:134–141
Es-haghi A, Hosseini SM, Khoshhesab ZM (2012) Development and application of a new solid-phase microextraction fiber by sol–gel technology on titanium wire. Anal Chim Acta 742:74–79
Hu X, Mu L, Zhou Q, Wen J, Pawliszyn J (2011) ssDNA aptamer-based column for simultaneous removal of nanogram per liter level of illicit and analgesic pharmaceuticals in drinking water. Environ Sci Technol 45:4890–4895
Mccooeye MA, Ells B, Barnett DA, Purves RW, Guevremont R (2001) Quantitation of morphine and codeine in human urine using high-field asymmetric waveform ion mobility spectrometry (FAIMS) with mass spectrometric detection. J Anal Toxicol 25:81–87
Zhang X, Chen M, Cao G, Hu G (2013) Determination of morphine and codeine in human urine by gas chromatography-mass spectrometry. J Anal Methods Chem 1155:1–6
Broussard LA, Presley LC, Pittman T, Clouette R, Wimbish GH (1997) Simultaneous identification and quantitation of codeine, morphine, hydrocodone, and hydromorphone in urine as trimethylsilyl and oxime derivatives by gas chromatography–mass spectrometry. Clin Chem 43(6):1029–1032
Coles R, Kushnir MM, Nelson GJ, Mcmillin GA, Urry FM (2007) Simultaneous determination of codeine, morphine, hydrocodone, hydromorphone, oxycodone, and 6-acetylmorphine in urine, serum, plasma, whole blood, and meconium by LC-MS-MS. J Anal Toxicol 31:1–14
He H, Shay SD, Caraco Y, Wood M, Wood AJJ (1998) Simultaneous determination of codeine and it seven metabolites in plasma and urine by high-performance liquid chromatography with ultraviolet and electrochemical detection. J Chromatogr B 708:185–193
Hyotylainen T, Siren H, Riekkola M-L (1996) Determination of morphine analogues, caffeine and amphetamine in biological fluids by capillary electrophoresis with the marker technique. J Chromatogr A 735:439–447
Acknowledgments
The authors are grateful for financial support of this work by the Research Council of Isfahan University of Technology and the Center of Excellency in Chemistry of Isfahan University of Technology. The authors thank the Iran National Science Foundation (grant no. 93030712) for its support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hashemian, Z., Khayamian, T. & Saraji, M. Anticodeine aptamer immobilized on a Whatman cellulose paper for thin-film microextraction of codeine from urine followed by electrospray ionization ion mobility spectrometry. Anal Bioanal Chem 407, 1615–1623 (2015). https://doi.org/10.1007/s00216-014-8392-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8392-5