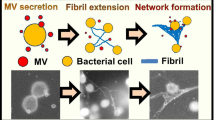
Abstract
We established an optimized biofilm observation method using a hydrophilic ionic liquid (IL), 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]). In the present study, a biofilm was formed by Staphylococcus epidermidis. Using field emission (FE) scanning electron microscopy (SEM) and transmission electron microscopy (TEM), the colonization of assemblages formed by microbial cells was observed as a function of the cultivation time. FE-TEM analysis revealed that the fibril comprises three types of protein. In addition, the ultrastructure of each protein monomer was visualized. It was expected that the curly-structured protein plays an important role in extension during fibril formation. Compared to the conventional sample preparation method for electron microscopy, a fine structure was easily obtained by the present method using IL. This observation technique can provide valuable information to characterize the ultrastructure of the fibril and biofilm that has not been revealed till date. Furthermore, these findings of the molecular architecture of the fibril and the colonization behavior of microbial cells during biofilm formation are useful for the development of antibacterial drugs and microbial utilization.







Similar content being viewed by others
References
Franson TR, Sheth NK, Rose HD, Sohnle PG (1984) Scanning electron microscopy of bacteria adherent to intravascular catheters. J Clin Microbiol 20:500–505
Kolari M, Schmidt U, Kuismanen E, Salkinoja-Salonen MS (2002) Firm but slippery attachment of Deinococcus geothermalis. J Bacreriol 184:2473–2480
Donlan RM (2002) Biofilms and device-associated infections. Emerg Infect Dis 7:277–281
Clauss M, Trampuz A, Borenz O, Bohner M, Ilchmann T (2010) Biofilm formation on bone grafts and bone graft substitutes: comparison of different materials by a standard in vitro test and microcalorimetry. Acta Biomater 6:3791–3797
Dankert J, Hogt AH, Feijen J (1986) Biomedical polymers: bacterial adhesion, colonization, and infection. Crit Rev Biocompat 2:219–301
An YH, Friedman RJ (1998) Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res 43:338–348
Ishii S, Koki J, Unno H, Hori K (2004) Two morphological types of cell appendages on a strongly adhesive bacterium, Acinetobacter sp. strain Tol 5. Appl Environ Microbiol 70:5026–5029
Schmid T, Burkhard J, Yeo BS, Zhang W, Zenobi R (2008) Towards chemical analysis of nanostructures in biofilms I: imaging of biological nanostructures. Anal Bioanal Chem 391:1899–1905
Mandlik A, Swierczynski A, Das A, Ton-That H (2008) Pili in gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol 16:33–40
Hilleringmann M, Ringler P, Muller SA, Angelis GD, Rappuoli R et al (2009) Molecular architecture of Streptococcus pneumoniae TIGR4 pili. EMBO J 28:3921–3930
Banner MA, Cunniffe JG, Macintosh RL, Foster TJ, Rohde H et al (2007) Localized tufts of fibrils on Staphylococcus epidermidis NCTC 11047 are comprised of the accumulation-associated protein. J Bacteriol 189:2793–2804
Mack D, Siemssen N, Laufs R (1992) Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun 60:2048–2057
Otto M (2009) Staphylococcus epidermidis—the ‘accidental’ pathogen. Nat Rev Microbiol 7:555–567
Nelson A, Hultenby K, Hell E, Riedel HM, Brismar H et al (2009) Staphylococcus epidermidis isolated from newborn infants express pilus-like structures and are inhibited by the cathelicidin-derived antimicrobial peptide LL37. Pediatr Res 66:174–178
Macintosh RL, Brittan JL, Bhattacharya R, Jenkinson HF, Derrick J et al (2009) The terminal A domain of the fibrillar accumulation-associated protein (Aap) of Staphylococcus epidermidis mediates adhesion to human corneocytes. J Bacteriol 191:7007–7016
Saka H, Mima T, Takeuchi Y, Marukawa T, Arai S et al (2006) Observation of gas-solid and gas-liquid reactions by in-situ environmental holder in TEM. Microsc Microanal 12:764–765
Takahashi C, Yaakob Y, Yusop MZM, Kalita G, Tanemura M (2014) Direct observation of structural change in Au-incorporated carbon nanofibers during field emission process. Carbon 75:277–280
Haider M, Uhlemann S, Schwan E, Rose H, Kabius B, Urban K (1998) Electron microscopy image enhanced. Nat 392:768–769
Yamasaki J, Kawai T, Tanaka N (2004) Direct observation of a stacking fault in Si1-xGex semiconductors by spherical aberration-corrected TEM and conventional ADF-STEM. J Electron Microsc 53:129–135
Mangel S, Aronovitch E, Enyashin AN, Houben L, Bar-Sadan M (2014) Atomic-scale evolution of a growing core-shell nanoparticle. J Am Chem Soc 136:12564–12567
Zakharov VV, Mosevitsky MI (2010) Oligomeric structure of brain abundant proteins GAP-43 and BASP1. J Struct Biol 170:470–483
Mahmoudi M, Shokrgozar MA, Sardari S, Moghadam MK, Vali H, Laurentc S, Stroeved P (2011) Irreversible changes in protein conformation due to interaction with superparamagnetic iron oxide nanoparticles. Nanoscale 3:1127–1138
Wang Y, Xu J, Wanga Y, Chen H (2013) Emerging chirality in nanoscience. Chem Soc Rev 42:2930–2962
Wilkes JS, Zaworotko MJ (1992) Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J Chem Soc Chem Commun 965–967
Torimoto T, Okazaki K, Kiyama T, Hirahara K, Tanaka N et al (2006) Sputter deposition onto ionic liquids: simple and clean synthesis of highly dispersed ultrafine metal nanoparticles. Appl Phys Lett 89:243117
Takahashi C, Shirai T, Fuji M (2012) Observation of interactions between hydrophilic ionic liquid and water on wet agar gels by FE-SEM and its mechanism. Mater Chem Phys 133:565–572
Kuwabata S, Tsuda T, Torimoto T (2010) Room-temperature ionic liquids. A new medium for material production and analyses under vacuum conditions. J Phys Chem Lett 1:3177–3188
Ishigaki Y, Nakamura Y, Takehara T, Nemoto N, Kurihara T et al (2011) Comparative study of hydrophilic and hydrophobic ionic liquids for observing cultured human cells by scanning electron microscopy. Microsc Res Tech 74:415–420
Takahashi C, Shirai T, Fuji M (2013) FE-SEM observation of swelled seaweed using hydrophilic ionic liquid; 1-butyl-3-methylimidazolium tetrafluoroborate. Microsco Res Tech 76:66–71
Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D et al (1996) Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol 20:1083–1091
O’Gara JP (2007) Ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett 270:179–188
Dubey GP, Yehuda SB (2011) Intercellular nanotubes mediate bacterial communication. Cell 144:590–600
Davis DM, Sowinski S (2008) Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol 9:431–436
Characklis WG, McFeters GA, Marshall KC (1990) In: Characklis WG, Marshall KC (eds) Biofilms. Wiley, New York
Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G (1997) A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun 65:519–524
Pei L, Flock JI (2001) Functional study of antibodies against a fibrogenin-binding protein in Staphylococcus epidermidis adherence to polyethylene catheters. J Infect Dis 184:52–55
Potter A, Ceotto H, Giambiagi-Demarval M, dos Santos KR, Nes IF et al (2009) The gene bap, involved in biofilm production, is present in Staphylococcus spp. strains from nosocomial infections. J Microbiol 47:319–326
Christner M, Franke GC, Schommer NN, Wendt U, Wegert K et al (2010) The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin. Mol Microbiol 75:187–207
Mack D, Davies AP, Harris LG, Jeeves R, Pascoe B et al (2013) In: Moriarty F, Zaat SAJ, Busscher HJ (eds) Staphylococcus epidermidis in biomaterial-associated infections. Springer, New York
Acknowledgments
This study was partially supported by the research grant from the Institute of Pharmaceutical Life Sciences, Aichi Gakuin University. The authors are grateful to Dr. Y. Morita of Aichi Gakuin University, Japan, for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 258 kb)
Rights and permissions
About this article
Cite this article
Takahashi, C., Kalita, G., Ogawa, N. et al. Electron microscopy of Staphylococcus epidermidis fibril and biofilm formation using image-enhancing ionic liquid. Anal Bioanal Chem 407, 1607–1613 (2015). https://doi.org/10.1007/s00216-014-8391-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8391-6