Abstract
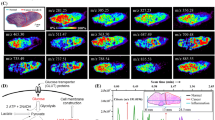
Mass spectrometry imaging (MSI) allows for the direct and simultaneous analysis of the spatial distribution of molecular species from sample surfaces such as tissue sections. One of the goals of MSI is monitoring the distribution of compounds at the cellular resolution in order to gain insights about the biology that occurs at this spatial level. Infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) imaging of cervical tissue sections was performed using a spot-to-spot distance of 10 μm by utilizing the method of oversampling, where the target plate is moved by a distance that is less than the desorption radius of the laser. In addition to high spatial resolution, high mass accuracy (±1 ppm) and high mass resolving power (140,000 at m/z = 200) were achieved by coupling the IR-MALDESI imaging source to a hybrid quadrupole Orbitrap mass spectrometer. Ion maps of cholesterol in tissues were generated from voxels containing <1 cell, on average. Additionally, the challenges of imaging at the cellular level in terms of loss of sensitivity and longer analysis time are discussed.

Cellular-level mass spectrometry imaging using IR-MALDESI and oversampling




Similar content being viewed by others
References
Tanaka K, Waki H, Ido Y et al (1988) Protein and polymer analyses up to m/z 100,000 by laser ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 2:151–153
Karas M, Hillenkamp F (1988) Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem 60:2299–2301
Fenn JB, Mann M, Meng CK et al (1989) Electrospray ionization for mass spectrometry of large biomolecules. Science 246:64–71
Aebersold R, Mann M (2003) Mass spectrometry-based proteomics. Nature 422:198–207. doi:10.1038/nature01511
Gupta MN, Roy I (2004) Enzymes in organic media. Forms, functions and applications. Eur J Biochem 271:2575–2583. doi:10.1111/j.1432-1033.2004.04163.x
Caprioli RM, Farmer TB, Gile J (1997) Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem 69:4751–4760
Nittler L, Delcorte A, Bertrand P, Migeon H-N (2011) Investigating the relation between the secondary yield enhancement and the structure of the metallic overlayer in metal-assisted SIMS. Surf Interface Anal 43:103–106. doi:10.1002/sia.3425
Lebec V, Boujday S, Poleunis C et al (2014) Time-of-flight secondary ion mass spectrometry investigation of the orientation of adsorbed antibodies on SAMs correlated to biorecognition tests. J Phys Chem C 118:2085–2092. doi:10.1021/jp410845g
Norris JL, Caprioli RM (2013) Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem Rev 113:2309–2342. doi:10.1021/cr3004295
Laiko VV, Baldwin MA, Burlingame AL (2000) Atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem 72:652–657
Goodwin RJ (2012) Sample preparation for mass spectrometry imaging: small mistakes can lead to big consequences. J Proteome 75:4893–4911. doi:10.1016/j.jprot.2012.04.012
Takáts Z, Wiseman JM, Gologan B, Cooks RG (2004) Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 306:471–473. doi:10.1126/science.1104404
Cody RB, Laramée J, Durst HD (2005) Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem 77:2297–2302. doi:10.1021/ac050162j
McEwen CN, McKay RG, Larsen BS (2005) Analysis of solids, liquids, and biological tissues using solids probe introduction at atmospheric pressure on commercial LC/MS instruments. Anal Chem 77:7826–7831. doi:10.1021/ac051470k
Sampson JS, Hawkridge AM, Muddiman DC (2006) Generation and detection of multiply-charged peptides and proteins by matrix-assisted laser desorption electrospray ionization (MALDESI) Fourier transform ion cyclotron resonance mass spectrometry. J Am Soc Mass Spectrom 17:1712–1716. doi:10.1016/j.jasms.2006.08.003
Eikel D, Vavrek M, Smith S et al (2011) Liquid extraction surface analysis mass spectrometry (LESA-MS) as a novel profiling tool for drug distribution and metabolism analysis: the terfenadine example. Rapid Commun Mass Spectrom 25:3587–3596. doi:10.1002/rcm.5274
Robichaud G, Barry JA, Garrard KP, Muddiman DC (2013) Infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) imaging source coupled to a FT-ICR mass spectrometer. J Am Soc Mass Spectrom 24:92–100. doi:10.1007/s13361-012-0505-9
Robichaud G, Barry JA, Muddiman DC (2014) IR-MALDESI Mass Spectrometry Imaging of Biological Tissue Sections Using Ice as a Matrix. J Am Soc Mass Spectrom 25:319–328. doi:10.1007/s13361-013-0787-6
Berkenkamp S, Karas M, Hillenkamp F (1996) Ice as a matrix for IR-matrix-assisted laser desorption/ionization: mass spectra from a protein single crystal. Proc Natl Acad Sci U S A 93:7003–7007
Pirkl A, Soltwisch J, Draude F, Dreisewerd K (2012) Infrared matrix-assisted laser desorption/ionization orthogonal-time-of-flight mass spectrometry employing a cooling stage and water ice as a matrix. Anal Chem 84:5669–5676. doi:10.1021/ac300840b
Nemes P, Vertes A (2007) Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Anal Chem 79:8098–8106. doi:10.1021/ac071181r
Garden RW, Sweedler JV (2000) Heterogeneity within MALDI samples as revealed by mass spectrometric imaging. Anal Chem 72:30–36
Spengler B, Hubert M (2002) Scanning microprobe matrix-assisted laser desorption ionization (SMALDI) mass spectrometry: instrumentation for sub-micrometer resolved LDI and MALDI surface analysis. J Am Soc Mass Spectrom 13:735–748
Koestler M, Kirsch D, Hester A et al (2008) A high-resolution scanning microprobe matrix-assisted laser desorption / ionization ion source for imaging analysis on an ion trap / Fourier transform ion cyclotron resonance mass spectrometer. Rapid Commun Mass Spectrom 22:3275–3285. doi:10.1002/rcm
Soltwisch J, Goritz G, Jungmann JH et al (2014) MALDI mass spectrometry imaging in microscope mode with infrared lasers: bypassing the diffraction limits. Anal Chem 86:321–325
Heeren RM (2014) Getting the picture: the coming of age of imaging MS. Int J Mass Spectrom. doi:10.1016/j.ijms.2014.04.021
Jurchen JC, Rubakhin SS, Sweedler JV (2005) MALDI-MS imaging of features smaller than the size of the laser beam. J Am Soc Mass Spectrom 16:1654–1659. doi:10.1016/j.jasms.2005.06.006
Kampmeier J, Dreisewerd K, Schurenberg M, Strupat K (1997) Investigations of 2, 5-DHB and succinic acid as matrices for IR and UV MALDI. Part : I UV and IR laser ablation in the MALDI process. Int J Mass Spectrom Ion Process 176:31–41
Olsen JV, de Godoy LMF, Li G et al (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol Cell Proteomics 4:2010–2021. doi:10.1074/mcp. T500030-MCP200
Kessner D, Chambers M, Burke R et al (2008) ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics 24:2534–2536. doi:10.1093/bioinformatics/btn323
Robichaud G, Garrard KP, Barry JA, Muddiman DC (2013) MSiReader: an open-source interface to view and analyze high resolving power MS imaging files on Matlab platform. J Am Soc Mass Spectrom 24:718–721. doi:10.1007/s13361-013-0607-z
Rogowitz BE, Treinish LA (1998) Data visualization: the end of the rainbow. IEEE Spectr 35:52–59
Light A, Bartlein PJ (2004) The end of the rainbow? Color schemes for improved data graphics. EOS Trans Am Geophys Union 85:385–391. doi:10.1029/2004EO400002
Borland D, Taylor MR (2007) Rainbow color map (still) considered harmful. IEEE Comput Graph Appl 27:14–17
Barry J, Robichaud G, Bokhart MT et al (2014) Mapping antiretroviral drugs in tissue by IR-MALDESI MSI coupled to the Q Exactive and comparison with LC-MS/MS SRM assay. J Am Soc Mass Spectrom 25:2038–2047. doi:10.1007/s13361-014-0884-1
Brunzel NA (2004) Fundamentals of urine & body fluid analysis, 2nd edn. Saunders, Philadelphia, PA
Acknowledgments
The authors thank Professor Angela D. M. Kashuba and her group from UNC Eshelman School of Pharmacy for providing the cervical tissues samples. The authors also gratefully acknowledge the financial assistance received from the National Institutes of Health (R01GM087964), the W.M. Keck foundation, and North Carolina State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Mass Spectrometry Imaging with guest editors Andreas Römpp and Uwe Karst.
Rights and permissions
About this article
Cite this article
Nazari, M., Muddiman, D.C. Cellular-level mass spectrometry imaging using infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) by oversampling. Anal Bioanal Chem 407, 2265–2271 (2015). https://doi.org/10.1007/s00216-014-8376-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8376-5