Abstract
Wild-type T4 bacteriophage and recombinant reporter lac Z T4 bacteriophage carrying the β-galactosidase gene were used for detection of generic Escherichia coli by monitoring the release of β-galactosidase upon phage-mediated cell lysis. The reaction was performed on a paper-based portable culture device to limit the diffusion of reagents and, hence, increase the sensitivity of the assay, and to avoid handling large sample volumes, making the assay suitable for on-site analysis. Chromogenic (chlorophenol red-β-d-galactopyranoside, CPRG) and bioluminescent (6-O-β-galactopyranosyl-luciferin, Beta-Glo®) β-galactosidase substrates were tested in the assay. Water samples were first filtered through 0.45-μm pore size filters to concentrate bacteria. The filters were then placed into the paper-based device containing nutrient medium and incubated at 37 °C for 4 h. Bacteriophage with the respective indicator substrate was added to the device, and signal (color, luminescence) development was recorded with a digital camera, luminometer, or luminescence imaging device. It was demonstrated that as low as 40 or <10 colony-forming units (cfu) ml−1 of E. coli can be detected visually within 8 h when wild-type T4 bacteriophage or recombinant lacZ T4 bacteriophage were used in the assay, respectively. Application of the bioluminescent β-galactosidase substrate allowed reliable detection of <10 cfu ml−1 within 5.5 h. The specificity of the assay was demonstrated using a panel of microorganisms including Aeromonas hydrophila, Enterobacter cloacae, E. coli, and Salmonella Typhimurium.
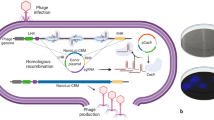
Scheme for rapid E. coli assay including filtration of water sample, short incubation on the filter in a paper-based culture device, addition of bacteriophage and [beta]-galactosidase substrate, and recording/processing of the accumulated color or luminescence signal.







Similar content being viewed by others
References
Yager P, Domingo GJ, Gerdes J (2008) In: Annual review of biomedical engineering, vol 10. Annual review of biomedical engineering. Annual Reviews, Palo Alto, pp 107–144
Center for Disease Control and Prevention (2012) Global WASH-related diseases and contaminants. http://www.cdc.gov/healthywater/wash_diseases.html. Accessed Jan 25 2014
Boubetra A, Le Nestour F, Allaert C, Feinberg M (2011) Appl Environ Microbiol 77(10):3360–3367
Kleinheinz GT, Busse KM, Gorman W, McDermott CM (2012) Lake Res Manag 28(4):328–337
US Environmental Protection Agency (2012) Recreational water quality control. http://water.epa.gov/scitech/swguidance/standards/criteria/health/recreation/index.cfm. Accessed Jan 25 2014
Byrne B, Stack E, Gilmartin N, O’Kennedy R (2009) Sensors 9(6):4407–4445
Aldus CF, van Amerongen A, Ariens RMC, Peck MW, Wichers JH, Wyatt GM (2003) J Appl Microbiol 95(2):380–389
Heo J, Hua SZ (2009) Sensors 9(6):4483–4502
Brunt J, Webb MD, Peck MW (2010) Appl Environ Microbiol 76(13):4143–4150
Foudeh AM, Didar TF, Veres T, Tabrizian M (2012) Lab Chip 12(18):3249–3266
Kronlein MR, Stedtfeld RD, Sorensen J, Bhaduri P, Stedtfeld T, Eanes S, Harichandran V, Haynes K, Stevens M, Hashsham SA (2013) Water Environ Res 85(10):889–916
Mandeville R, Griffiths M, Goodridge L, McIntyre L, Ilenchuk TT (2003) Anal Lett 36(15):3241–3259
Mao CB, Liu AH, Cao BR (2009) Angew Chem Int Ed 48(37):6790–6810
Hagens S, Loessner MJ (2007) Appl Microbiol Biotechnol 76(3):513–519
Brovko LY, Anany H, Griffiths MW (2012) Adv Food Nutr Res 67:241–288
Tolba M, Minikh O, Brovko LY, Evoy S, Griffiths MW (2010) Appl Environ Microbiol 76(2):528–535
Minikh O, Tolba M, Brovko LY, Griffiths MW (2010) J Microbiol Meth 82(2):177–183
Wu Y, Brovko L, Griffiths MW (2001) Lett Appl Microbiol 33(4):311–315
Smartt AE, Ripp S (2011) Anal Bioanal Chem 400(4):991–1007
Ulitzur N, Ulitzur S (2006) Appl Environ Microbiol 72(12):7455–7459
Waddell TE, Poppe C (2000) FEMS Microbiol Lett 182:285–289
Sarkis GJ, Jacobs WR, Hatfull GF (1995) Mol Microbiol 15(6):1055–1067
Awais R, Fukudomi H, Miyanaga K, Unno H, Tanji Y (2006) Biotech Prog 22(3):853–859
Oda M, Morita M, Unno H, Tanji Y (2004) Appl Environ Microbiol 70(1):527–534
Goodridge L, Griffiths M (2002) Food Res Int 35(9):863–870
Khoury MK, Parker I, Aswad DW (2010) Anal Biochem 397(1):129–131
Martinez AW, Phillips ST, Butte MJ, Whitesides GM (2007) Angew Chem Int Ed 46(8):1318–1320
Martinez AW, Phillips ST, Whitesides GM (2008) Proc Natl Acad Sci U S A 105(50):19606–19611
Ellerbee AK, Phillips ST, Siegel AC, Mirica KA, Martinez AW, Striehl P, Jain N, Prentiss M, Whitesides GM (2009) Anal Chem 81(20):8447–8452
Kutter E (2009) In: Clokie MRJ, Kropinski AM (eds) Methods in molecular biology, vol 501. Methods in molecular biology. Humana Press Inc, Totowa, USA
New DC, Miller-Martini DM, Wong YH (2003) Phytother Res 17(5):439–448
Goodridge L (2007) USA Patent 7,244,612
Anany H, Lingohr EJ, Villegas A, Ackermann HW, She YM, Griffiths MW, Kropinski AM (2011) Virol J 8. doi:24210.1186/1743-422x-8-242
Funes-Huacca M, Wu A, Szepesvari E, Rajendran P, Kwan-Wong N, Razgulin A, Shen Y, Kagira J, Campbell R, Derda R (2012) Lab Chip 12(21):4269–4278
Edberg SC, Rice EW, Karlin RJ, Allen MJ (2000) J Appl Microbiol 88:106S–116S
Wohlsen T, Bayliss J, Bates J, Gray B, Johnson S, Schneider P (2008) J Wat Supply Res Technol AQUA 57(8):569–576
Brenner KP, Rankin CC, Sivaganesan M (1996) J Microbiol Meth 27(2–3):111–119
Hallas G, Giglio S, Capurso V, Monis PT, Grooby WL (2008) J Appl Microbiol 105(4):1138–1149
McLain JET, Williams CF (2008) Water Res 42(15):4041–4048
Reznikoff WS (1992) Mol Microbiol 6(17):2419–2422
Acknowledgments
This work was supported by the NSERC Sentinel, Bioactive Paper Research Network.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Analytical Bioluminescence and Chemiluminescence with guest editors Elisa Michelini and Mara Mirasoli.
Rights and permissions
About this article
Cite this article
Burnham, S., Hu, J., Anany, H. et al. Towards rapid on-site phage-mediated detection of generic Escherichia coli in water using luminescent and visual readout. Anal Bioanal Chem 406, 5685–5693 (2014). https://doi.org/10.1007/s00216-014-7985-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7985-3