Abstract
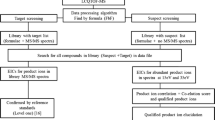
The continuing emergence of designer drugs imposes high demands on the scope and sensitivity of toxicological drug screening procedures. An ultra-high performance liquid chromatography/high-resolution time-of-flight mass spectrometry (UHPLC-HR-TOFMS) method was developed for screening and simultaneous confirmation of both designer drugs and other drugs of abuse in urine samples in a single run. The method covered selected synthetic cannabinoids and cathinones, amphetamines, natural cannabinoids, opioids, cocaine and other important drugs of abuse, together with their main urinary metabolites. The database consisted of 277 compounds with molecular formula and exact monoisotopic mass; retention time was included for 192 compounds, and primary and secondary qualifier ion exact mass for 191 and 95 compounds, respectively. Following a solid-phase extraction, separation was performed by UHPLC and mass analysis by HR-TOFMS. MS, and broad-band collision-induced dissociation data were acquired at m/z range 50–700. Compound identification was based on a reverse database search with acceptance criteria for retention time, precursor ion mass accuracy, isotopic pattern and abundance of qualifier ions. Mass resolving power in spiked urine samples was on average FWHM 23,500 and mass accuracy 0.3 mDa. The mean and median cut-off concentrations determined for 75 compounds were 4.2 and 1 ng/mL, respectively. The range of cut-off concentrations for synthetic cannabinoids was 0.2–60 ng/mL and for cathinones 0.7–15 ng/mL. The method proved to combine high sensitivity and a wide scope in a manner not previously reported in drugs of abuse screening. The method’s feasibility was demonstrated with 50 authentic urine samples.

Extracted ion chromatograms of metabolites of synthetic cannabinoids and their fragments, including a new common metabolite: JWH-072-propanoic acid


Similar content being viewed by others

References
Favretto D, Pascali JP, Tagliaro F (2013) New challenges and innovation in forensic toxicology. Focus on the "new psychoactive substances". J Chromatogr A 1287:84–95
Huffman JW, Zengin G, Wu M-J, Lu J, Hynd G, Bushell K, Thompson ALS, Bushell S, Tartal C, Hurts DP, Reggio PH, Selley DE, Cassidy MP, Wiley JL, Martin BR (2005) Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg Med Chem 13:89–112
Hermanns-Clausen M, Kneisel S, Szabo B, Auwärter V (2012) Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction 108:534–544
European Monitoring Centre for Drugs and Drug Addiction (2009) EMCDDA 2009 Thematic paper, Understanding the ‘spice’ phenomenon.
Airuehia E, Young-Walker L, Nittler J (2012) A review of bath salts: evolving designer drugs of abuse. J Clinic Toxicol 2:125. doi:10.4172/2161-0495.1000125
Fass JA, Fass AD, Garcia AS (2012) Synthetic cathinones (bath salts): legal status and patterns of abuse. Ann Pharmacother 46:436–441
Broecker S, Herre S, Wüst B, Zweigenbaum J, Pragst F (2011) Development and practical application of a library of CID accurate mass spectra of more than 2,500 toxic compounds for systematic toxicological analysis by LC-QTOF-MS with data-dependent acquisition. Anal Bioanal Chem 400:101–117
Lee HK, Ho CS, Iu YPH, Lai PSJ, Shek CC, Lo Y-C, Klinke HB, Wood M (2009) Development of a broad toxicological screening technique for urine using ultra-performance liquid chromatography and time-of-flight mass spectrometry. Anal Chim Acta 649:80–90
Guale F, Shahreza S, Walterscheid JP, Chen H-H, Arndt C, Kelly AT, Mozayani A (2013) Validation of LC-TOF-MS screening for drugs, metabolites, and collateral compounds in forensic toxicology specimens. J Anal Toxicol 37:17–24
Wissenbach DK, Meyer MR, Remane D, Philipp AA, Weber AA, Maurer HH (2011) Drugs of abuse screening in urine as part of a metabolite-based LC-MSn screening concept. Anal Bioanal Chem 400:3481–3489
Welter J, Meyer MR, Wolf EU, Weinmann W, Kavanagh P, Maurer HH (2013) 2-Methiopropamine, a thiophene analogue of methamphetamine: studies on its metabolism and detectability in the rat and human using GC-MS and LC-(HR)-MS techniques. Anal Bioanal Chem 10:3125–3135
de Castro A, Gergov M, Östman P, Ojanperä I, Pelander A (2012) Combined drug screening and confirmation by liquid chromatography time-of-flight mass spectrometry with reverse database search. Anal Bioanal Chem 403:1265–1278
Ammann J, McLaren JM, Gerostamoulos D, Beyer J (2012) Detection and quantification of new designer drugs in human blood: part 1—synthetic cannabinoids. J Anal Toxicol 36:372–380
Kneisel S, Auwärter V (2012) Analysis of 30 synthetic cannabinoids in serum by liquid chromatography-electrospray ionization tandem mass spectrometry after liquid–liquid extraction. J Mass Spectrom 47:825–835
Dresen S, Kneisel S, Weinmann W, Zimmermann R, Auwärter V (2011) Development and validation of liquid chromatography-tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. J Mass Spectrom 46:163–171
Teske J, Weller J-P, Fieguth A, Rothämel T, Schulz Y, Tröger HD (2010) Sensitive and rapid quantification of the cannabinoid receptor agonist naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in human serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B 878:2659–2663
Hutter M, Kneisel S, Auwärter V, Neukamm MA (2012) Determination of 22 synthetic cannabinoids in human hair by liquid chromatography-tandem mass spectrometry. J Chromatogr B 903:95–101
Salomone A, Gerace E, D'Urso F, Di Corcia D, Vincenti M (2012) Simultaneous analysis of several synthetic cannabinoids, THC, CBD and CBN, in hair by ultra-performance liquid chromatography tandem mass spectrometry method validation and application to real samples. J Mass Spectrom 47:604–610
Coulter C, Garnier M, Moore C (2011) Synthetic cannabinoids in oral fluid. J Anal Toxicol 35:424–430
Strano-Rossi S, Anzillotti L, Castrignano E, Romolo FS, Chiarotti M (2012) Ultra high performance liquid chromatography-electrospray ionization-tandem mass spectrometry screening method for direct analysis of designer drugs, "spice" and stimulants in oral fluid. J Chromatogr, A 1258:37–42
Kneisel S, Auwärter V, Kempf J (2012) Analysis of 30 synthetic cannabinoids in oral fluid using liquid chromatography-electrospray ionization tandem mass spectrometry. Drug Test Analysis. doi:10.1002/dta.1429
Kneisel S, Speck M, Moosmann B, Corneillie TM, Butlin NG, Auwärter V (2013) LC/ESI-MS/MS method for quantification of 28 synthetic cannabinoids in neat oral fluid and its application to preliminary studies on their detection windows. Anal Bioanal Chem 405:4691–4706
Hutter M, Broecker S, Kneisel S, Auwärter V (2012) Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in ´herbal mixtures´using LC-MS/MS techniques. J Mass Spectrom 47:54–65
Möller I, Wintermeyer A, Bender K, Jübner M, Thomas A, Krug O, Schänzer W, Thevis M (2010) Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test Analysis 3:609–620
Moran CL, Le V-H, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, Kennedy PD, Endres GW, Ciske FL, Kramer JB, Kornilov AM, Bratton LD, Dobrowolski PJ, Wessinger WD, Fantegrossi WE, Prather PL, James LP, Radominska-Pandya A, Moran JH (2010) Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem 83:4228–4236
Sobolevsky T, Prasolov I, Rodchenkov G (2010) Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int 200:141–147
de Jager AD, Warner JV, Henman M, Ferguson W, Hall A (2012) LC-MS/MS method for the quantitation of metabolites of eight commonly-used synthetic cannabinoids in human urine—an Australian perspective. J Chromatogr B 897:22–31
Ammann J, Drummer OH, Gerostamoulos D, Beyer J (2011) Detection of synthetic cannabinoids in biological samples—a review. Tiaft Bulletin 41:21–27
Baselt RC (2002) Disposition of toxic drugs and chemicals in man, 6th edn. Biomedical Publications, California
Zanchetti G, Floris I, Piccinotti A, Tameni S, Pollettini A (2012) Rapid and robust confirmation and quantification of 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THC-COOH) in urine by column switching LC-MS-MS analysis. J Mass Spectrom 47:124–130
Gergov M, Boucher B, Ojanperä I, Vuori E (2001) Toxicological screening of urine for drugs by liquid chromatography/time-of-flight mass spectrometry with automated target library search based on elemental formulas. Rapid Commun Mass Spectrom 15:521–526
Ojanperä I, Kolmonen M, Pelander A (2012) Current use of high-resolution mass spectrometry in drug screening relevant to clinical and forensic toxicology and doping control. Anal Bioanal Chem 403:1203–1220
Liotta E, Gottardo R, Bertaso A, Pollettini A (2010) Screening for pharmaco-toxicologically relevant compounds in biosamples using high-resolution mass spectrometry: a 'metabolomic' approach to the discrimination between isomers. J Mass Spectrom 45:261–271
Laks S, Pelander A, Vuori E, Ali-Tolppa E, Sippola E, Ojanperä I (2004) Analysis of street drugs in seized material without primary reference standards. Anal Chem 76:7375–7379
Ojanperä S, Pelander A, Pelzing M, Krebs I, Vuori E, Ojanperä I (2006) Isotopic pattern and accurate mass determination in urine drug screening by liquid chromatography/time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 20:1161–1167
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC-MS/MS. Anal Chem 75:3019–3030
Wissenbach DK, Meyer MR, Remane D, Weber AA, Maurer HH (2011) Development of the first metabolite-based LC-MSn urine drug screening procedure-exemplified for antidepressants. Anal Bioanal Chem 400:79–88
Saleh A, Stephanson NN, Granelli I, Villen T, Beck O (2012) Evaluation of a direct high-capacity target screening approach for urine drug testing using liquid chromatography-time-of-flight mass spectrometry. J Chromatogr B 909:6–13
Lovett DP, Yanes EG, Herbelin TW, Knoerzer TA, Levisky JA (2013) Structure elucidation and identification of a common metabolite for naphthoylindole-based synthetic cannabinoids using LC-TOF and comparison to a synthetic reference standard. Forensic Sci Int 226:81–87
Shevyrin IV, Melkozerov V, Nevero A, Eltsov O, Morzherin Y, Shafran Y (2013) Identification and analytical properties of new synthetic cannabimimetics bearing 2,2,3,3-tetramethylcyclopropanecarbonyl moiety. Forensic Sci Int 226:62–73
Tyrkkö E, Pelander A, Ojanperä I (2010) Differentiation of structural isomers in a target drug database by LC/Q-TOFMS using fragmentation prediction. Drug Test Analysis 2:259–270
Acknowledgements
The authors would thank the EU Commission (JUST/2009/DPIP/AG/0948 and JUST/2011/DPIP/AG/3597) for its financial support for the projects ‘Spice and synthetic cannabinoids’ as well as ‘Spice II Plus’.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2.02 MB)
Rights and permissions
About this article
Cite this article
Sundström, M., Pelander, A., Angerer, V. et al. A high-sensitivity ultra-high performance liquid chromatography/high-resolution time-of-flight mass spectrometry (UHPLC-HR-TOFMS) method for screening synthetic cannabinoids and other drugs of abuse in urine. Anal Bioanal Chem 405, 8463–8474 (2013). https://doi.org/10.1007/s00216-013-7272-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7272-8