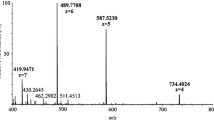
Abstract
Urine samples have been the predominant matrix for doping controls for several decades. However, owing to the complementary information provided by blood (as well as serum or plasma and dried blood spots (DBS)), the benefits of its analysis have resulted in continuously increasing appreciation by anti-doping authorities. On the one hand, blood samples allow for the detection of various different methods of blood doping and the abuse of erythropoiesis-stimulating agents (ESAs) via the Athlete Biological Passport; on the other hand, targeted and non-targeted drug detection by means of chromatographic–mass spectrometric methods represents an important tool to increase doping control frequencies out-of-competition and to determine drug concentrations particularly in in-competition scenarios. Moreover, blood analysis seldom requires in-depth knowledge of drug metabolism, and the intact substance rather than potentially unknown or assumed metabolic products can be targeted. In this review, the recent developments in human sports drug testing concerning mass spectrometry-based techniques for qualitative and quantitative analyses of therapeutics and emerging drug candidates are summarized and reviewed. The analytical methods include both low and high molecular mass compounds (e.g., anabolic agents, stimulants, metabolic modulators, peptide hormones, and small interfering RNA (siRNA)) determined from serum, plasma, and DBS using state-of-the-art instrumentation such as liquid chromatography (LC)–high resolution/high accuracy (tandem) mass spectrometry (LC-HRMS), LC–low resolution tandem mass spectrometry (LC-MS/MS), and gas chromatography–mass spectrometry (GC-MS).


Similar content being viewed by others
References
Donike M (2011) The detection of doping by means of chromatographic methods. 1966. Drug Test Anal 3:15–17
Catlin DH, Fitch KD, Ljungqvist A (2008) Medicine and science in the fight against doping in sport. J Intern Med 264:99–114
Segura J (2009) Is anti-doping analysis so far from clinical, legal or forensic targets? The added value of close relationships between related disciplines. Drug Test Anal 1:479–484
The World Anti-Doping Code - International Standard for Laboratories (2009) World Anti-Doping Agency. http://www.wada-ama.org/rtecontent/document/International_Standard_for_Laboratories_v6_0_January_2009.pdf. Accessed 24 Nov 2008
de Pencier C (1994) In: Hemmersbach P, Birkeland K (eds) Blood samples in doping control. Allkopi, Oslo, pp 3–16
Donike M (1976) The detection of doping agents in blood. Br J Sports Med 10:147–154
Donike M, Geyer H, Gotzmann A, Horning S, Mareck-Engelke U, Nitschke R, Nolteernsting E, Rauth S, Schänzer W, Seinsch I, Sigmund G (1994) In: Hemmersbach P, Birkeland K (eds) Blood samples in doping control. Allkopi, Oslo, pp 75–92
Birkeland K, Donike M, Ljungqvist A, Fagerhol M, Jensen J, Hemmersbach P, Oftebro H, Haug E (1997) Blood sampling in doping control. First experiences from regular testing in athletics. Int J Sports Med 18:8–12
Saugy M, Robinson N, Saudan C (2009) The fight against doping: back on track with blood. Drug Test Anal 1:474–478
Jelkmann W, Lundby C (2011) Blood doping and its detection. Blood 118:2395–2404
Pottgiesser T, Echteler T, Sottas PE, Umhau M, Schumacher YO (2012) Hemoglobin mass and biological passport for the detection of autologous blood doping. Med Sci Sports Exerc 44:835–843
Pottgiesser T, Sottas PE, Echteler T, Robinson N, Umhau M, Schumacher YO (2011) Detection of autologous blood doping with adaptively evaluated biomarkers of doping: a longitudinal blinded study. Transfusion 51:1707–1715
Segura J, Ventura R, Pascual JA (2011) Current strategic approaches for the detection of blood doping practices. Forensic Sci Int 213:42–48
Sottas PE, Robinson N, Saugy M (2010) The athlete's biological passport and indirect markers of blood doping. Handb Exp Pharmacol 195:305–326
Giraud S, Robinson N, Mangin P, Saugy M (2008) Scientific and forensic standards for homologous blood transfusion anti-doping analyses. Forensic Sci Int 179:23–33
Nelson M, Cooper S, Nakhla S, Smith S, King M, Ashenden MJ (2004) Validation of a test designed to detect blood-doping of elite athletes by homologous transfusion. Aust J Exp Biol Med Sci 25:27–33
Voss SC, Thevis M, Schinkothe T, Schanzer W (2007) Detection of homologous blood transfusion. Int J Sports Med 28:633–637
Bidlingmaier M, Manolopoulou J (2010) Detecting growth hormone abuse in athletes. Endocrinol Metab Clin North Am 39:25–32, vii
Bidlingmaier M, Strasburger CJ (2010) Growth hormone. Handb Exp Pharmacol 195:187–200
Holt RIG (2009) Detecting growth hormone abuse in athletes. Drug Test Anal 1:426–433
Guha N, Erotokritou-Mulligan I, Burford C, Strobridge G, Brigg J, Drake T, Bassett EE, Cowan D, Bartlett C, Sonksen PH, Holt RI (2010) Serum insulin-like growth factor-I and pro-collagen type III N-terminal peptide in adolescent elite athletes: implications for the detection of growth hormone abuse in sport. J Clin Endocrinol Metab 95:2969–2976
Holt RI, Erotokritou-Mulligan I, McHugh C, Bassett EE, Bartlett C, Fityan A, Bacon JL, Cowan DA, Sonksen PH (2010) The GH-2004 project: the response of IGF1 and type III pro-collagen to the administration of exogenous GH in non-Caucasian amateur athletes. Eur J Endocrinol 163:45–54
Holt RI (2011) Detecting growth hormone abuse in athletes. Anal Bioanal Chem 401:449–462
Erotokritou-Mulligan I, Guha N, Stow M, Bassett EE, Bartlett C, Cowan DA, Sonksen PH, Holt RI (2012) The development of decision limits for the implementation of the GH-2000 detection methodology using current commercial insulin-like growth factor-I and amino-terminal pro-peptide of type III collagen assays. Growth Hormon IGF Res 22:53–58
Thevis M, Kuuranne T, Geyer H, Schänzer W (2011) Annual banned-substance review: analytical approaches in human sports drug testing. Drug Test Anal 3:1–14
Thevis M, Kuuranne T, Geyer H, Schänzer W (2012) Annual banned-substance review: analytical approaches in human sports drug testing. Drug Test Anal 4:2–16
Barroso O, Handelsman DJ, Strasburger C, Thevis M (2012) Analytical challenges in the detection of peptide hormones for anti-doping purposes. Bioanalysis 4:1577–1590
Thevis M, Thomas A, Schänzer W (2011) Doping control analysis of selected peptide hormones using LC-MS(/MS). Forensic Sci Int 213:35–41
World Anti-Doping Agency (2013) The 2013 Prohibited List. http://www.wada-ama.org/en/World-Anti-Doping-Program/Sports-and-Anti-Doping-Organizations/International-Standards/Prohibited-List/. Accessed 7 Jan 2013
Gam LH, Tham SY, Latiff A (2003) Immunoaffinity extraction and tandem mass spectrometric analysis of human chorionic gonadotropin in doping analysis. J Chromatogr B 792:187–196
Lund H, Lovsletten K, Paus E, Halvorsen TG, Reubsaet L (2012) Immuno-MS based targeted proteomics: highly specific, sensitive, and reproducible human chorionic gonadotropin determination for clinical diagnostics and doping analysis. Anal Chem 84:7926–7932
Lund H, Snilsberg AH, Paus E, Halvorsen TG, Hemmersbach P, Reubsaet L (2013) Sports drug testing using immuno-MS: clinical study comprising administration of human chorionic gonadotropin to males. Anal Bioanal Chem 405:1569–1576
Baume N, Steel G, Edwards T, Thorstensen E, Miller BF (2008) No variation of physical performance and perceived exertion after adrenal gland stimulation by synthetic ACTH (Synacthen) in cyclists. Eur J Appl Physiol 104:589–600
Thevis M, Bredehöft M, Geyer H, Kamber M, Delahaut P, Schänzer W (2006) Determination of Synacthen in human plasma using immunoaffinity purification and liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 20:3551–3556
Chaabo A, de Ceaurriz J, Buisson C, Tabet JC, Lasne F (2011) Simultaneous quantification and qualification of Synacthen in plasma. Anal Bioanal Chem 399:1835–1843
Sanchis-Gomar F, Martinez-Bello VE, Nascimento AL, Perez-Quilis C, Garcia-Gimenez JL, Vina J, Gomez-Cabrera MC (2010) Desmopresssin and hemodilution: implications in doping. Int J Sports Med 31:5–9
Thomas A, Solymos E, Schänzer W, Baume N, Saugy M, Dellanna F, Thevis M (2011) Determination of vasopressin and desmopressin in urine by means of liquid chromatography coupled to quadrupole time-of-flight mass spectrometry for doping control purposes. Anal Chim Acta 707:107–113
Esposito S, Deventer K, T'Sjoen G, Vantilborgh A, Van Eenoo P (2013) Doping control analysis of desmopressin in human urine by LC-ESI-MS/MS after urine delipidation. Biomed Chromatogr 27:240–245
Esposito S, Deventer K, T'Sjoen G, Vantilborgh A, Delbeke FT, Goessaert AS, Everaert K, Van Eenoo P (2012) Qualitative detection of desmopressin in plasma by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 402:2789–2796
Bobin S, Popot MA, Bonnaire Y, Tabet JC (2001) Approach to the determination of insulin-like-growth-factor-I (IGF-I) concentration in plasma by high-performance liquid chromatography-ion trap mass spectrometry: use of a deconvolution algorithm for the quantification of multiprotonated molecules in electrospray ionization. Analyst 126:1996–2001
Nelson RW, Nedelkov D, Tubbs KA, Kiernan UA (2004) Quantitative mass spectrometric immunoassay of insulin like growth factor 1. J Proteome Res 3:851–855
Bredehöft M, Schänzer W, Thevis M (2008) Quantification of human insulin-like growth factor-1 and qualitative detection of its analogues in plasma using liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom 22:477–485
Kay R, Barton C, Ratcliffe L, Matharoo-Ball B, Brown P, Roberts J, Teale P, Creaser C (2008) Enrichment of low molecular weight serum proteins using acetonitrile precipitation for mass spectrometry based proteomic analysis. Rapid Commun Mass Spectrom: RCM 22:3255–3260
Kay RG, Barton C, Velloso CP, Brown PR, Bartlett C, Blazevich AJ, Godfrey RJ, Goldspink G, Rees R, Ball GR, Cowan DA, Harridge SD, Roberts J, Teale P, Creaser CS (2009) High-throughput ultra-high-performance liquid chromatography/tandem mass spectrometry quantitation of insulin-like growth factor-I and leucine-rich alpha-2-glycoprotein in serum as biomarkers of recombinant human growth hormone administration. Rapid Commun Mass Spectrom 23:3173–3182
Möller I, Thomas A, Geyer H, Schänzer W, Thevis M (2011) Synthesis, characterisation, and mass spectrometric detection of a pegylated EPO-mimetic peptide for sports drug testing purposes. Rapid Commun Mass Spectrom 25:2115–2123
Möller I, Thomas A, Wingender A, Machnik M, Schänzer W, Thevis M (2012) Detection of peginesatide in equine serum using liquid chromatography-tandem mass spectrometry for doping control purposes. Eur J Mass Spectrom 18:407–412
Stokes S (2012) Colombian doctor Beltrán Nino arrested with AICAR and TB-500 doping products. In: Velonation. http://www.velonation.com. Accessed 26 Jun 2013
Esposito S, Deventer K, Goeman J, Van der Eycken J, Van Eenoo P (2012) Synthesis and characterization of the N-terminal acetylated 17–23 fragment of thymosin beta 4 identified in TB-500, a product suspected to possess doping potential. Drug Test Anal 4:733–738
Thomas A, Schänzer W, Delahaut P, Thevis M (2012) Immunoaffinity purification of peptide hormones prior to liquid chromatography–mass spectrometry in doping controls. Methods 56:230–235
Thevis M, Thomas A, Schänzer W (2008) Mass spectrometric determination of insulins and their degradation products in sports drug testing. Mass Spectrom Rev 27:35–50
Thevis M, Thomas A, Schänzer W (2010) Insulin. Handb Exp Pharmacol 195:209–226
Staub A, Rudaz S, Saugy M, Veuthey JL, Schappler J (2010) Analysis of hemoglobin-based oxygen carriers by CE-UV/Vis and CE-ESI-TOF/MS. Electrophoresis 31:1241–1247
Thevis M, Thomas A, Kohler M, Beuck S, Schänzer W (2009) Emerging drugs: mechanism of action, mass spectrometry and doping control analysis. J Mass Spectrom 44:442–460
Menzies KJ, Hood DA (2012) The role of SirT1 in muscle mitochondrial turnover. Mitochondrion 12:5–13
Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J (2008) Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8:347–358
Höppner S, Schänzer W, Thevis M (2013) Fragmentation studies of SIRT1-activating drugs and their detection in human plasma for doping control purposes. Rapid Commun Mass Spectrom 27:35–50
Thevis M, Beuck S, Thomas A, Kortner B, Kohler M, Rodchenkov G, Schänzer W (2009) Doping control analysis of emerging drugs in human plasma - identification of GW501516, S-107, JTV-519, and S-40503. Rapid Commun Mass Spectrom 23:1139–1146
Thomas A, Guddat S, Kohler M, Krug O, Schänzer W, Petrou M, Thevis M (2010) Comprehensive plasma-screening for known and unknown substances in doping controls. Rapid Commun Mass Spectrom 24:1124–1132
Davidson BL, McCray PB Jr (2011) Current prospects for RNA interference-based therapies. Nat Rev Genet 12:329–340
Kurreck J (2009) RNA interference: from basic research to therapeutic applications. Angew Chem Int Ed Engl 48:1378–1398
Kohler M, Schänzer W, Thevis M (2011) RNA interference for performance enhancement and detection in doping control. Drug Test Anal 3:661–667
Kohler M, Thomas A, Walpurgis K, Schänzer W, Thevis M (2010) Detection of siRNA from plasma samples by mass spectrometry for doping control purposes. Anal Bioanal Chem 398:1305–1312
Huestis MA, Cone EJ (1998) Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol 22:445–454
Huestis MA, Mazzoni I, Rabin O (2011) Cannabis in sport: anti-doping perspective. Sports Med 41:949–966
Mareck U, Haenelt N, Geyer H, Guddat S, Kamber M, Brenneisen R, Thevis M, Schänzer W (2009) Temporal indication of cannabis use by means of THC glucuronide determination. Drug Test Anal 1:505–510
Schwope DM, Karschner EL, Gorelick DA, Huestis MA (2011) Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem 57:1406–1414
Huestis MA, Elsohly M, Nebro W, Barnes A, Gustafson RA, Smith ML (2006) Estimating time of last oral ingestion of cannabis from plasma THC and THCCOOH concentrations. Ther Drug Monit 28:540–544
Brenneisen R, Meyer P, Chtioui H, Saugy M, Kamber M (2010) Plasma and urine profiles of Delta9-tetrahydrocannabinol and its metabolites 11-hydroxy-delta9-tetrahydrocannabinol and 11-nor-9-carboxy-delta9-tetrahydrocannabinol after cannabis smoking by male volunteers to estimate recent consumption by athletes. Anal Bioanal Chem 396:2493–2502
Thevis M, Thomas A, Schänzer W (2011) Current role of LC-MS(/MS) in doping control. Anal Bioanal Chem 401:405–420
Schwope DM, Scheidweiler KB, Huestis MA (2011) Direct quantification of cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 401:1273–1283
Peng SH, Segura J, Farre M, de la Torre X (2000) Oral testosterone administration detected by testosterone glucuronidation measured in blood spots dried on filter paper. Clin Chem 46:515–522
Thomas A, Geyer H, Guddat S, Schänzer W, Thevis M (2011) Dried blood spots (DBS) for doping control analysis. Drug Test Anal 3:806–813
Thomas A, Geyer H, Schänzer W, Crone C, Kellmann M, Moehring T, Thevis M (2012) Sensitive determination of prohibited drugs in dried blood spots (DBS) for doping controls by means of a benchtop quadrupole/Orbitrap mass spectrometer. Anal Bioanal Chem 403:1279–1289
Cox HD, Rampton J, Eichner D (2013) Quantification of insulin-like growth factor-1 in dried blood spots for detection of growth hormone abuse in sport. Anal Bioanal Chem 405:1949–1958
Möller I, Thomas A, Geyer H, Schänzer W, Thevis M (2012) Development and validation of a mass spectrometric detection method of peginesatide in dried blood spots for sports drug testing. Anal Bioanal Chem 403:2715–2724
Ding J, List EO, Okada S, Kopchick JJ (2009) Perspective: proteomic approach to detect biomarkers of human growth hormone. Growth Hormon IGF Res 19:399–407
Reichel C (2011) OMICS-strategies and methods in the fight against doping. Forensic Sci Int 213:20–34
Kay RG, Creaser CS (2010) Application of mass spectrometry-based proteomics techniques for the detection of protein doping in sports. Expert Rev Proteome 7:185–188
Kohler M, Thomas A, Puschel K, Schänzer W, Thevis M (2009) Identification of human pituitary growth hormone variants by mass spectrometry. J Proteome Res 8:1071–1076
Walpurgis K, Kohler M, Thomas A, Wenzel F, Geyer H, Schänzer W, Thevis M (2012) Storage-induced changes of the cytosolic red blood cell proteome analyzed by 2D DIGE and high-resolution/high-accuracy MS. Proteomics 12:3263–3272
Horie M, Kawashima Y, Naka A, Matsumoto K, Kodera Y, Maeda T, Iida K (2011) Proteomic profiling of k-11706 responsive proteins. Int J Sports Med 32:559–564
Acknowledgments
The work was supported by the Federal Ministry of the Interior of the Federal Republic of Germany (Bonn, DE) and Antidoping Switzerland (Berne, CH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Anti-doping Analysis with guest editor Christopher Harrison.
Rights and permissions
About this article
Cite this article
Thevis, M., Thomas, A. & Schänzer, W. Targeting prohibited substances in doping control blood samples by means of chromatographic–mass spectrometric methods. Anal Bioanal Chem 405, 9655–9667 (2013). https://doi.org/10.1007/s00216-013-7224-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7224-3