Abstract
This manuscript discusses the application and the comparison between three statistical regression methods for handling data: parametric, nonparametric, and weighted regression (WR). These data were obtained from different chemometric methods applied to the high-performance liquid chromatography response data using the internal standard method. This was performed on a model drug Acyclovir which was analyzed in human plasma with the use of ganciclovir as internal standard. In vivo study was also performed. Derivative treatment of chromatographic response ratio data was followed by convolution of the resulting derivative curves using 8-points sin x i polynomials (discrete Fourier functions). This work studies and also compares the application of WR method and Theil's method, a nonparametric regression (NPR) method with the least squares parametric regression (LSPR) method, which is considered the de facto standard method used for regression. When the assumption of homoscedasticity is not met for analytical data, a simple and effective way to counteract the great influence of the high concentrations on the fitted regression line is to use WR method. WR was found to be superior to the method of LSPR as the former assumes that the y-direction error in the calibration curve will increase as x increases. Theil's NPR method was also found to be superior to the method of LSPR as the former assumes that errors could occur in both x- and y-directions and that might not be normally distributed. Most of the results showed a significant improvement in the precision and accuracy on applying WR and NPR methods relative to LSPR.

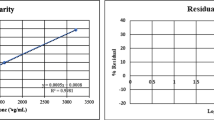
Comparison between RSD (percent) and E r (percent) calculated for Acyclovir, for area, D 1, and D 1/FF in the internal standard methods, using the three types of regression models: parametric, nonparametric, and weighted






Similar content being viewed by others
References
Miller JN, Miller JC (2002) Statistics and chemometrics for analytical chemistry, 4th edn. Prentice Hall, Harlow, pp 170–172
Nagaraja NV, Paliwal JK, Gupta RC (1999) Choosing the calibration model in assay validation. J Pharm Biomed Anal 20:433–438
Sadray S, Rezaee S, Rezakhah S (2003) Non-linear heteroscedastic regression model for determination of methotrexate in human plasma by high-performance liquid chromatography. J Chromatogr B 787:293–302
Almeida AM, Castel-Branco MM, Falcão AC (2002) Linear regression for calibration lines revisited: weighting schemes for bioanalytical methods. J Chromatogr B 774:215–222
Zeng QC, Zhang E, Dong H, Tellinghuisen J (2008) Weighted least squares in calibration: estimating data variance functions in high-performance liquid chromatography. J Chromatogr A 1206:147–152
Castillo MA, Castells RC (2001) Initial evaluation of quantitative performance of chromatographic methods using replicates at multiple concentrations. J Chromatogr A 921:121–133
Saari E, Perämäki P, Jalonen J (2008) Measurement uncertainty in the determination of total petroleum hydrocarbons (TPH) in soil by GC-FID. Chemom Intell Lab Sys 92:3–12
Kasiari M, Gikas E, Georgakakou S, Kazanis M, Panderi I (2008) Selective and rapid liquid chromatography/negative-ion electrospray ionization mass spectrometry method for the quantification of valacyclovir and its metabolite in human plasma. J Chromatogr B 864:78–86
Colombo S, Guignard N, Marzolini C, Telenti A, Biollaz J, Decosterd LA (2004) Determination of the new HIV-protease inhibitor atazanavir by liquid chromatography after solid-phase extraction. J Chromatogr B 810:25–34
Nascimentoa RS, Froesb RES, Silva NOC, Naveirab RLP, Mendesc DBC, Netoa WB, Silva JBB (2010) Comparison between ordinary least squares regression and weighted least squares regression in the calibration of metals present in human milk determined by ICP-OES. Talanta 80:1102–1109
Perrottet N, Beguin A, Meylan P, Pascual M, Manuel O, Buclin T, Biollaz J, Decosterd LA (2007) Determination of aciclovir and ganciclovir in human plasma by liquid chromatography-spectrofluorimetric detection and stability studies in blood samples. J Chromatogr B 852:420–429
Korany MA, Fahmy OT, Mahgoub H, Maher HM (2005) Non-parametric linear regression of discrete Fourier transform convoluted chromatographic peak responses in non-ideal conditions. Talanta 66:1073–1087
Korany MA, Hewala II, Abdel-Hai KM (2006) Non-parametric linear regression of discrete Fourier transform convoluted densitometric peak responses. J Pharm Biomed Anal 40:1048–1056
Maher HM, Youssef RM (2008) Simultaneous determination of ternary drug mixtures using square wave polarography subjected to non-parametric and chemometric peak convolution. Chemom Intell Lab Sys 94:95–103
Korany MA, Maher HM, Galal SM, Ragab MAA (2010) Non-parametric linear regression of discrete Fourier transform convoluted chromatographic peak responses under non-ideal conditions of internal standard method. Talanta 83:93–109
Ouyanga LQ, Wua HL, Niea JF, Zhanga Y, Zoua HY, Fua HY, Yua RQ (2009) Simultaneous determination of psoralen and isopsoralen in plasma and Chinese medicine Xian Ling Gu Bao capsule by using HPLC-DAD coupled with alternating trilinear decomposition algorithm. Anal Chim Acta 650:160–166
Canada-Canadaa F, Arancibiab JA, Escandarb GM, Ibanezb GA, Espinosa Mansillaa A, Munoz de la Penaa A, Olivieri AC (2009) Second-order multivariate calibration procedures applied to high-performance liquid chromatography coupled to fast-scanning fluorescence detection for the determination of fluoroquinolones. J Chromatogr A 1216:4868–4876
Pap TL, Pápai Z (2001) Application of a new mathematical function for describing chromatographic peaks. J Chromatogr A 930:53–60
Vivó-Truyols G, Torres-Lapasió JR, van Nederkassel AM, Vander Heyden Y, Massart DL (2005) Automatic program for peak detection and deconvolution of multi-overlapped chromatographic signals part I: peak detection. J Chromatogr A 1096:133–145
Moffat AC, Osselton MD, Widdop B (2004) Clark's analysis of drugs and poisons, 3rd edn. Pharmaceutical, London
Studenberg SD, Long JD, Woolf JH, Bruner CJ, Wilson D, Woolley JL (1995) A robotics-based liquid chromatographic assay for the measurement of atovaquone in plasma. J Pharm Biomed Anal 13:1383–1393
Gaillard Y, Prévosto JM, Cheminel V, Soares O, Chaulet JF (1995) New solid-phase extraction for an improved high-performance liquid chromatographic procedure for the quantitation of halofantrine and monodesbutylhalofantrine in blood or plasma. J Chromatogr B: Biomed Sci Appl 668:315–321
Malma M, Bergqvista Y (2007) Determination of eflornithine enantiomers in plasma, by solid-phase extraction and liquid chromatography with evaporative light-scattering detection. J Chromatogr B 846:98–104
Dao YJ, Jiao Z, Zhong MK (2008) Simultaneous determination of aciclovir, ganciclovir, and penciclovir in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr B 867:270–276
Palmberger TF, Hombach J, Bernkop-Schnürch A (2008) Thiolated chitosan: development and in vitro evaluation of an oral delivery system for acyclovir. Int J Pharm 348:54–60
Tzanavaras PD, Themelis DG (2007) High-throughput HPLC assay of acyclovir and its major impurity guanine using a monolithic column and a flow gradient approach. J Pharm Biomed Anal 43:1526–1530
Bahrami GH, Mirzaeei SH, Kiani A (2005) Determination of acyclovir in human serum by high-performance liquid chromatography using liquid–liquid extraction and its application in pharmacokinetic studies. J Chromatogr B 816:327–331
Dubhashi SS, Vavia PR (2000) HPTLC method to study skin permeation of acyclovir. J Pharm Biomed Anal 23:1017–1022
Ayad MM, Abdellatef HE, El-Henawee MM, El-Sayed HM (2007) Spectrophotometric and spectrofluorimetric methods for analysis of acyclovir and acebutolol hydrochloride. Spectrochim Acta Part A 66:106–110
Abdellatef HE, El-Henawee MM, El-Sayed HM, Ayad MM (2006) Spectrophotometric and spectrofluorimetric methods for analysis of acyclovir and acebutolol hydrochloride. Spectrochim Acta Part A 65:997–999
Basavaiah K, Prameela HC (2002) Simple spectrophotometric determination of acyclovir in bulk drug and formulations. Il Farmaco 57:443–449
Fernández M, Sepúlveda J, Aránguiz T, Plessing C (2003) Technique validation by liquid chromatography for the determination of acyclovir in plasma. J Chromatogr B 791:357–363
Riley MR, Kastrup EK, Hebel SK (2001) Drug facts and comparisons, 55th edn. A Wolters Kluwer Company, St. Louis
Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG (1996) Goodman and Gillman's the pharmacological basis of therapeutics, 9th edn
Armitage P, Berry G (1994) Statistical methods in medical research, 3rd edn. Blackwell Scientific, Oxford
Oppenheimer L, Capizzi TP, Weppeiman RM, Mehta H (1983) Determining the lowest limit of reliable assay measurement. Anal Chem 55:638–643
Acknowledgement
The authors are thankful to Pharaonia Pharmaceuticals (Alexandria, Egypt) for supplying the raw materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korany, M.A., Maher, H.M., Galal, S.M. et al. Comparative study of some robust statistical methods: weighted, parametric, and nonparametric linear regression of HPLC convoluted peak responses using internal standard method in drug bioavailability studies. Anal Bioanal Chem 405, 4835–4848 (2013). https://doi.org/10.1007/s00216-013-6859-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6859-4