Abstract
The present study dealt with the forced degradation behaviour of rosuvastatin under ICH prescribed stress conditions. The drug was found to be labile under acid hydrolytic and photolytic conditions, while it was stable to base/neutral hydrolytic, oxidative and thermal stress. In total, 11 degradation products were formed, which were separated on a C-18 column using a stability-indicating method. LC-MS analyses indicated that five degradation products had the same molecular mass as that of the drug, while the remaining six had 18 Da less than the drug. Structure elucidation of all the degradation products was executed using sophisticated and modern structural characterization tools, viz. LC-MS/TOF, LC-MSn, on-line H/D exchange and LC-NMR. The degradation pathway and mechanisms of degradation of the drug were delineated. Additionally, in silico toxicity was predicted for all the degradation products using TOPKAT and DEREK software and compared with the drug. This study demonstrates a comprehensive approach of degradation studies during the drug development phase.

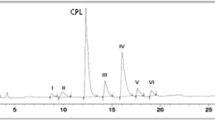
Degradation pathway of rosuvastatin







Similar content being viewed by others
References
ICH (2003) Stability testing of new drug substances and products Q1A(R2). International Conference on Harmonisation, IFPMA, Geneva
WHO (2009) Stability testing of active pharmaceutical ingredients and finished pharmaceutical products. Technical Report Series No. 953. World Health Organization, Geneva
Singh S, Handa T, Narayanam M, Sahu A, Junwal M, Shah RP (2012) J Pharm Biomed Anal 69:148–173
ICH (2009) Pharmaceutical development Q8(R2). International Conference on Harmonisation, IFPMA, Geneva
Gao T, Liu Y, Ji Y, Wu X, Xu J (2012) J Pharm Biomed Anal 66:381–386
Holčapek M, Jirásko R, Lísa M (2012) J Chromatogr A 1259:3–15
Mehta S, Shah RP, Priyadarshi R, Singh S (2010) J Pharm Biomed Anal 52(3):345–354
Modhave DT, Handa T, Shah RP, Singh S (2011) J Pharm Biomed Anal 56:538–545
Shah RP, Sahu A, Singh S (2011) Eur J Chem 2:152–157
Sturm S, Seger C (2012) J Chromatogr A 1259:50–61
Thomas S, Joshi SC, Vir D, Agarwal A, Rao RD, Sridhar I, Xavier CM, Mathela CS (2012) J Pharm Biomed Anal 63:112–119
Jackson EK (2001) In: Brunton LL (ed) Goodman & Gilman's the pharmacological basis of therapeutics, 10th edn. McGraw-Hill, New York
Gao J, Peart TE, Syoboda L, Backus S (2007) J Chromatogr B 856:35–40
Hussain S, Patel H, Tan A (2009) Bioanalysis 1:529–535
Kallem RR, Karthik A, Chakradhar L, Mullangi R, Srinivas NR (2007) Arzneim Forsch 57:705–711
Tian L, Jiang J, Huang Y (2007) Chin Pharm J 42:1174–1177
Xu DH, Ruan ZR, Zhou Q, Yuan H, Jiang B (2006) Rapid Commun Mass Spectrom 20:2369–2375
Zhang H, Liang C, Cheng XH, Chen YJ, Li YY, Xiong YQ (2006) Chin J New Drugs Clin Remedies 25:909–914
Krishnaiah C, Murthy MV, Prasad BJD, Satyanarayana B, Kumar RKM (2009) Anal Chem: Indian J 8:277–283
Mehta TN, Patel AK, Kulkarni GM, Suubbaiah G (2005) J AOAC Int 88:1142–1147
Raj HA, Rajput SJ, Dave JB, Patel CN (2009) Int J ChemTech Res 1:677–689
Gajjar AKK, Shah VDD (2010) Eurasian J Anal Chem 5:280–298
Gomes FP, García PL, Alves JMP, Singh AK, Kedor-Hackmann ERM, Santoro MIRM (2009) Anal Lett 42:1784–1804
Astarita A, DellaGreca M, Lesce MR, Montanaro S, Previtera L, Temussi F (2007) J Photochem Photobiol A 187:263–268
Finkelstein N (2007) US Patent 7,244,844
Reddy GVR, Reddy BV, Haque SW, Gautam HD, Kumar P, Kumar AP, Park JH (2011) Quim Nova 34:250–255
Trivedi HK, Patel MC (2012) Sci Pharm 80:393–406
ICH (1996) Photostability testing of new drug substances and products Q1B. International Conference on Harmonization, IFPMA, Geneva
Bakshi M, Singh S (2002) J Pharm Biomed Anal 28:1011–1040
Singh S, Bakshi M (2000) Pharm Technol Online 24:1–14
Introduction to the preparation and properties of hydrogen peroxide. Laboratory of Inorganic Synthesis and Catalysis, Switzerland. http://lsci.epfl.ch/files/content/sites/lsci/files/load/perxoide-property.pdf. Accessed 22 Dec 2012
Shah RP, Kumar V, Singh S (2008) Rapid Commun Mass Spectrom 22:613–622
Shah RP, Kumar V, Singh S (2008) J Pharm Pharmacol 60:A9–A10
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 3.45 MB)
Rights and permissions
About this article
Cite this article
Shah, R.P., Sahu, A. & Singh, S. LC-MS/TOF, LC-MSn, on-line H/D exchange and LC-NMR studies on rosuvastatin degradation and in silico determination of toxicity of its degradation products: a comprehensive approach during drug development. Anal Bioanal Chem 405, 3215–3231 (2013). https://doi.org/10.1007/s00216-013-6760-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6760-1