Abstract
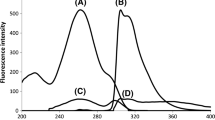
Chiral capillary electrophoresis method has been developed to separate aspartate and glutamate enantiomers to investigate the putative neuromodulator function of d-Asp in the central nervous system. To achieve appropriate detection sensitivity fluorescent derivatization with 4-fluoro-7-nitro-2,1,3-benzoxadiazole and laser-induced fluorescence detection was applied. Although, simultaneous baseline separation of the two enantiomer pairs could be achieved by using 3 mM 6-monodeoxy-6-mono(3-hydroxy)propylamino-β-cyclodextrin (HPA-β-CD), further improvement of the chemical selectivity was required because of the high excess of l-enantiomers in real samples to be analyzed. The system selectivity was fine-tuned by combination of 8 mM heptakis(2,6-di-O-methyl)-β-cyclodextrin and 5 mM HPA-β-CD in order to increase the resolution between aspartate and glutamate enantiomers. The method was validated for biological application. The limits of detection for d-Asp and d-Glu were 17 and 9 nM, respectively, while the limit of quantification for both analytes was 50 nM. This is the lowest quantification limit reported so far for NBD-tagged d-Asp and d-Glu obtained by validated capillary electrophoresis laser-induced fluorescence method. The applicability of the method was demonstrated by analyzing brain samples of 1-day-old chickens. In all the studied brain areas, the d-enantiomer contributed 1–2 % of the total aspartate content, corresponding to 17–45 nmol/g wet tissue.




Similar content being viewed by others
References
D’Aniello A, Guiditta A (1977) Identification of d-aspartic acid in the brain of Octopus vulgaris Lam. J Neurochem 29(6):1053–1057
D’Aniello A (2007) d-Aspartic acid: an endogenous amino acid with an important neuroendocrine role. Brain Res Rev 53(2):215–234
D’Aniello S, Somorjai I, Garcia-Fernandez J, Topo E, D'Aniello A (2011) d-Aspartic acid is a novel endogenous neurotransmitter. FASEB J 25(3):1014–1027
Kim PM, Duan X, Huang AS, Liu CY, Ming GL, Song H, Snyder SH (2010) Aspartate racemase, generating neuronal d-aspartate, regulates adult neurogenesis. Proc Natl Acad Sci U S A 107(7):3175–3179
Kirschner DL, Green TK (2009) Separation and sensitive detection of d-amino acids in biological matrices. J Sep Sci 32(13):2305–2318
Kitagawa F, Otsuka K (2011) Recent progress in capillary electrophoretic analysis of amino acid enantiomers. J Chromatogr B Analyt Technol Biomed Life Sci 879(29):3078–3095
Perry M, Li Q, Kennedy RT (2009) Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal Chim Acta 653(1):1–22
Lapainis T, Sweedler JV (2008) Contributions of capillary electrophoresis to neuroscience. J Chromatogr A 1184(1–2):144–158
Kirschner DL, Jaramillo M, Green TK (2007) Enantioseparation and stacking of cyanobenz[f]isoindole-amino acids by reverse polarity capillary electrophoresis and sulfated beta-cyclodextrin. Anal Chem 79(2):736–743
Miao H, Rubakhin SS, Sweedler JV (2005) Subcellular analysis of d-aspartate. Anal Chem 77(22):7190–7194
Thorsen G, Bergquist J (2000) Chiral separation of amino acids in biological fluids by micellar electrokinetic chromatography with laser-induced fluorescence detection. J Chromatogr B: Biomed Sci Appl 745(2):389–397
Wang S, Fan L, Cui S (2009) CE-LIF chiral separation of aspartic acid and glutamic acid enantiomers using human serum albumin and sodium cholate as dual selectors. J Sep Sci 32(18):3184–3190
Wagner Z, Tábi T, Zachar G, Csillag A, Szökő E (2011) Comparison of quantitative performance of three fluorescence labels in CE/LIF analysis of aspartate and glutamate in brain microdialysate. Electrophoresis 32(20):2816–2822
Puelles L, Martinez-de-la-Torre M, Paxinos G, Watson C, Martinez S (2007) The chick brain in stereotaxic coordinates: an atlas featuring neuromeric subdivisions and mammalian homologies. Academic, Amsterdam
Tábi T, Lohinai Z, Pálfi M, Levine M, Szökő E (2008) CE-LIF determination of salivary cadaverine and lysine concentration ratio as an indicator of lysine decarboxylase enzyme activity. Anal Bioanal Chem 391(2):647–651
US-FDA (2001) Guidance for industry—bioanalytical method validation.
Szökő E, Tábi T (2010) Analysis of biological samples by capillary electrophoresis with laser induced fluorescence detection. J Pharm Biomed Anal 53(5):1180–1192
Scriba GK (2008) Cyclodextrins in capillary electrophoresis enantioseparations—recent developments and applications. J Sep Sci 31(11):1991–2011
Huang Y, Shi M, Zhao S (2009) Quantification of d-Asp and d-Glu in rat brain and human cerebrospinal fluid by microchip electrophoresis. J Sep Sci 32(17):3001–3006
Samakashvili S, Ibanez C, Simo C, Gil-Bea FJ, Winblad B, Cedazo-Minguez A, Cifuentes A (2011) Analysis of chiral amino acids in cerebrospinal fluid samples linked to different stages of Alzheimer disease. Electrophoresis 32(19):2757–2764
Simo C, Barbas C, Cifuentes A (2002) Sensitive micellar electrokinetic chromatography-laser-induced fluorescence method to analyze chiral amino acids in orange juices. J Agric Food Chem 50(19):5288–5293
Song Y, Feng Y, LeBlanc MH, Zhao S, Liu YM (2006) Assay of trace d-amino acids in neural tissue samples by capillary liquid chromatography/tandem mass spectrometry. Anal Chem 78(23):8121–8128
Fillet M, Bechet I, Hubert P, Crommen J (1996) Resolution improvement by use of carboxymethyl-beta-cyclodextrin as chiral additive for the enantiomeric separation of basic drugs by capillary electrophoresis. J Pharm Biomed Anal 14(8–10):1107–1114
Lelievre F, Gareil P, Jardy A (1997) Selectivity in capillary electrophoresis: application to chiral separations with cyclodextrins. Anal Chem 69(3):385–392
Lurie IS, Klein RF, Dal Cason TA, LeBelle MJ, Brenneisen R, Weinberger RE (1994) Chiral resolution of cationic drugs of forensic interest by capillary electrophoresis with mixtures of neutral and anionic cyclodextrins. Anal Chem 66(22):4019–4026
Rudaz S, Geiser L, Souverain S, Prat J, Veuthey JL (2005) Rapid stereoselective separations of amphetamine derivatives with highly sulfated gamma-cyclodextrin. Electrophoresis 26(20):3910–3920
Galaverna G, Corradini R, Dossena A, Marchelli R, Vecchio G (1997) Histamine-modified beta-cyclodextrins for the enantiomeric separation of dansyl-amino acids in capillary electrophoresis. Electrophoresis 18(6):905–911
Ivanyi R, Jicsinszky L, Juvancz Z, Roos N, Otta K, Szejtli J (2004) Influence of (hydroxy)alkylamino substituents on enantioseparation ability of single-isomer amino-beta-cyclodextrin derivatives in chiral capillary electrophoresis. Electrophoresis 25(16):2675–2686
Guttman A, Cooke N (1994) Practical aspects of chiral separations of pharmaceuticals by capillary electrophoresis I. Separation optimization. J Chromatogr A 680(1):157–162
Rawjee YY, Williams RL, Vigh G (1994) Efficiency optimization in capillary electrophoretic chiral separations using dynamic mobility matching. Anal Chem 66(21):3777–3781
Chen F, Zhang S, Qi L, Chen Y (2006) Chiral capillary electrophoretic separation of amino acids derivatized with 9-fluorenylmethylchloroformate using mixed chiral selectors of beta-cyclodextrin and sodium taurodeoxycholate. Electrophoresis 27(14):2896–2904
Jin LJ, Rodriguez I, Li SF (1999) Enantiomeric separation of amino acids derivatized with fluoresceine isothiocyanate isomer I by micellar electrokinetic chromatography using beta- and gamma-cyclodextrins as chiral selectors. Electrophoresis 20(7):1538–1545
Fillet M, Chankvetadze B, Crommen J, Blaschke G (1999) Designed combination of chiral selectors for adjustment of enantioseparation selectivity in capillary electrophoresis. Electrophoresis 20(13):2691–2697
Fillet M, Hubert P, Crommen J (1997) Enantioseparation of nonsteroidal anti-inflammatory drugs by capillary electrophoresis using mixtures of anionic and uncharged beta-cyclodextrins as chiral additives. Electrophoresis 18(6):1013–1018
Jakubetz H, Juza M, Schurig V (1998) Dual chiral recognition system involving cyclodextrin derivatives in capillary electrophoresis II. Enhancement of enantioselectivity. Electrophoresis 19(5):738–744
Lelievre F, Gareil P, Bahaddi Y, Galons H (1997) Intrinsic selectivity in capillary electrophoresis for chiral separations with dual cyclodextrin systems. Anal Chem 69(3):393–401
Abushoffa AM, Fillet M, Hubert P, Crommen J (2002) Prediction of selectivity for enantiomeric separations of uncharged compounds by capillary electrophoresis involving dual cyclodextrin systems. J Chromatogr A 948(1–2):321–329
Tábi T, Magyar K, Szökő E (2003) Chiral characterization of deprenyl-N-oxide and other deprenyl metabolites by capillary electrophoresis using a dual cyclodextrin system in rat urine. Electrophoresis 24(15):2665–2673
Katane M, Homma H (2011) d-Aspartate—an important bioactive substance in mammals: a review from an analytical and biological point of view. J Chromatogr B Analyt Technol Biomed Life Sci 879(29):3108–3121
Acknowledgments
This work has been supported by the Hungarian National Scientific Research Fund (OTKA 63415 and 73219) and TÁMOP-4.2.1/B-09/1/KMR-2010-0001.
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagner, Z., Tábi, T., Jakó, T. et al. Chiral separation and determination of excitatory amino acids in brain samples by CE-LIF using dual cyclodextrin system. Anal Bioanal Chem 404, 2363–2368 (2012). https://doi.org/10.1007/s00216-012-6384-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6384-x