Abstract
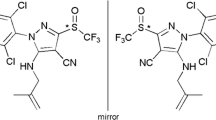
Herein is reported, for the first time, a simple and highly sensitive chiral high-performance liquid chromatography (HPLC) method for the simultaneous quantitative determination of difenoconazole stereoisomers and their hydroxylated metabolite difenoconazole alcohol (CGA-205375) enantiomers in vegetables and soil matrix. The separation of difenoconazole and CGA-205375 including their simultaneous enantioseparation was studied using four different polysaccharide-type chiral stationary phases (CSPs) in combination with n-hexane–polar organic alcohols mobile phase. Chiralcel OJ consisting of 25 % of cellulose tris(4-methylbenzoate) coated on wide-pore polysaccharide silica gel exhibited higher resolving ability compared to cellulose tris(3,5-dimethylphenylcarbamate) (Chiralcel OD) as well as to its similar amylose derivative (Chiralpak AD) CSPs for this particular set of chiral analytes. Baseline separation and simultaneous enantioseparation of difenoconazole and its metabolite CGA-205375 could be achieved under optimized separation conditions. Based on the established HPLC method, enantioselective analysis method for this fungicide and its main chiral metabolite in vegetables and soil matrix were developed and validated. Parameters including the matrix effect, linearity, precision, accuracy, and stability were evaluated. Under the optimal conditions, the mean recoveries from cucumber, tomato, and soil matrix ranged from 81.65 to 94.52 %, with relative standard deviations in the range of 1.05–8.32 % for all stereoisomers. Coefficients of determination R 2 ≥ 0.998 were achieved for each enantiomer in the cucumber, tomato and soil matrix calibration curves within the range of 0.5–50 μg mL-1. The limits of quantification for all enantiomers in three matrices were all below 0.1 μg mL-1. The methodology was successfully applied for simultaneous enantioselective analysis of difenoconazole stereoisomers and their metabolite in the real samples, indicating its efficacy in investigating the environmental stereochemistry of difenoconazole in food and environmental matrix.

Typical chromatograms of difenoconazole and its chiral metabolite CGA-205375 in real soil sample detected by chiral HPLC system and GC-MS





Similar content being viewed by others
References
Gal J (2008) Chirality 20:1072–1084
Smith SW (2009) Toxicol Sci 110:4–30
Williams A (1996) Pestic Sci 46:3–9
Zhou Y, Li L, Lin KD, Zhu XP, Liu WP (2009) Chirality 21:421–427
Liu W, Gan J, Schlenk D, Jury WA (2005) Proc Nat Acad Sci USA 102:701–706
Wong CS (2006) Anal Bioanal Chem 386:544–558
Wong CS, Lau F, Clark M, Mabury SA, Muir DCG (2002) Environ Sci Technol 36:1257–1262
Liu W, Gan J, Lee S, Werner I (2005) Environ Toxicol Chem 24:1861–1866
Elmarakby SA, Supplee D, Cook R (2001) J Agric Food Chem 49:5285–5293
Gopinath K, Radhakrishnan N, Jayaraj J (2006) Crop Prot 25:1024–1031
Vawdrey L, Grice K, Westerhuis D (2008) Australas Plant Path 37:552–558
Horsfield A, Wicks T, Davies K, Wilson D, Paton S (2010) Australas Plant Path 39:368–375
Dahmen H, Staub T (1992) Plant Dis 76:774–777
EFSA Journal (2011) 9: Article 1967
Sanderson JT, Boerma J, Lansbergen GWA, van den Berg M (2002) Toxicol Appl Pharm 182:44–54
Sanderson JT (2006) Toxicol Sci 94:3–21
Thom E, Ottow JCG, Benckiser G (1997) Environ Pollut 96:409–414
Banerjee K, Oulkar DP, Patil SH, Dasgupta S, Adsule PG (2008) Pest Manag Sci 64:283–289
Kahle M, Buerge IJ, Hauser A, MD Mu ller, Poiger T (2008) Environ Sci Technol 42:7193–7200
Mukhopadhyay S, Das S, Bhattacharyya A, Pal S (2011) B Environ Contam Tox 87:54–57
Garrison AW, Avants JK, Jones WJ (2011) Environ Sci Technol 45:2186–2193
Garrison AW (2006) Environ Sci Technol 40:16–23
Gadher P, Mercer E, Baldwin B, Wiggins T (1983) Pestic Biochem Phys 19:1–10
Kurihara N, Miyamoto J, Paulson G, Zeeh B, Skidmore M, Hollingworth R, Kuiper H (1997) Pure Appl Chem 69:2007–2025
Buerge IJ, Poiger T, Mueller MD, Buser HR (2006) Environ Sci Technol 40:5443–5450
Ye J, Wu J, Liu W (2009) TRAC Trend Anal Chem 28:1148–1163
Li J, Dong FS, Xu J, Liu XG, Li YB, Shan WL, Zheng YQ (2011) Anal Chim Acta 702:127–135
Chankvetadze B, Blaschke G (2001) J Chromatogr A 906:309–363
Chankvetadze B (1997) J Chromatogr A 792:269–295
Wu Y, Lee H, Li S (2001) J Chromatogr A 912:171–179
Garrison AW, Avants JK, Miller RD (2011) Int J Environ Res Public Health 8:3453–3467
Toribio L, Del Nozal M, Bernal J, Jiménez J, Alonso C (2004) J Chromatogr A 1046:249–253
Li J, Dong F, Xu J, Liu X, Li Y, Shan W, Zheng Y (2012) Chirality 24:294–302
Dong FS, Liu XG, Zheng YQ, Cao Q, Li CJ (2010) Chirality 22:292–298
Tanaka N, Kobayashi H, Ishizuka N, Minakuchi H, Nakanishi K, Hosoya K, Ikegami T (2002) J Chromatogr A 965:35–49
Chankvetadze B, Yamamoto C, Kamigaito M, Tanaka N, Nakanishi K, Okamoto Y (2006) J Chromatogr A 1110:46–52
Chankvetadze B, Yashima E, Okamoto Y (1994) J Chromatogr A 670:39–49
Lin K, Xu C, Zhou S, Liu W, Gan J (2007) Chirality 19:171–178
Lao W, Gan J (2006) J Chromatogr A 1117:184–193
Kunath A, Theil F, Wagner J (1994) J Chromatogr A 684:162–167
O’Brien T, Crocker L, Thompson R, Thompson K, Toma P, Conlon D, Feibush B, Moeder C, Bicker G, Grinberg N (1997) Anal Chem 69:1999–2007
Schenck FJ, Lehotay SJ (2000) J Chromatogr A 868:51–61
Schenck FJ, Lehotay SJ, Vega V (2002) J Sep Sci 25:883–890
Okihashi M, Kitagawa Y, Akutsu K, Obana H, Tanaka Y (2005) J Pestic Sci 30:368–377
Plössl F, Giera M, Bracher F (2006) J Chromatogr A 1135:19–26
González-Rodríguez RM, Rial-Otero R, Cancho-Grande B, Simal-Gándara J (2008) J Chromatogr A 1196:100–109
Acknowledgments
This work was financially supported by the Nature Science Foundation of China (NSFC, 31071706 and 31000863), the foundation of the National Basic Research Program of China (The 973 Program, Grant no. 2009CB119000) and Public Service Sector Research and Development Project (200903054 and 200903033). We are also grateful to Professor Bezhan Chankvetadze for providing professional review and helpful comments on the present paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Li and Fengshou Dong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, J., Dong, F., Cheng, Y. et al. Simultaneous enantioselective determination of triazole fungicide difenoconazole and its main chiral metabolite in vegetables and soil by normal-phase high-performance liquid chromatography. Anal Bioanal Chem 404, 2017–2031 (2012). https://doi.org/10.1007/s00216-012-6240-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6240-z