Abstract
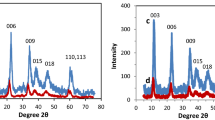
This paper demonstrates, for the first time, the great potential of using Zn/Al layered double hydroxide intercalated sodium dodecyl benzene sulfonate (Zn/Al-SDBS-LDH) as a solid-phase extraction (SPE) material in the extraction of persistent organic pollutants prior to the determination of gas chromatography–mass spectrometry in environmental water samples. Zn/Al-SDBS-LDH, a relatively inexpensive and simply prepared material, was synthesized and used as a SPE adsorbent to quantitatively determine the concentration of five polycyclic aromatic hydrocarbons (PAHs) in environmental water samples. Factors affecting extraction efficiency, such as, eluent type, eluent volume, flow rate of sample, sample volume, and amount of adsorbent, were investigated and optimized in detail. Experimental results indicate that there is an excellent linear relationship between peak area and the concentration of PAHs over the range of 5–500 ng L−1, and the precisions (relative standard deviation (RSD)) were 2.5–6.3 % under the optimum conditions. Based on the ratio of chromatographic signal-to-base line noise (S/N = 3), the limits of detection could reach 1.2–3.2 ng L−1. This novel method was successfully applied to the analysis of PAHs in environmental water samples. As such, we show here that the use of Zn/Al-SDBS-LDH as SPE adsorbent materials, coupled with gas chromatography–mass spectrometry, is an excellent improvement in the routine analysis of PAHs at trace levels in the environment.









Similar content being viewed by others
References
Zhu LZ, Cai XF, Wang J (2005) PAHs in aquatic sediment in Hangzhou, China: analytical methods, pollution pattern, risk assessment and sources. J Environ Sci 17(5):748–755
Ma JP, Xiao RH, Li JH, Yu JB, Zhang YQ, Chen LX (2010) Determination of 16 polycyclic aromatic hydrocarbons in environmental water samples by solid-phase extraction using multi-walled carbon nanotubes as adsorbent coupled with gas chromatography–mass spectrometry. J Chromatogr A 1217(34):5462–5469
Valavanidis A, Vlachogianni Th, Triantafillaki S, Dassenakis M, Androutsos F, Scoullos M (2008) Polycyclic aromatic hydrocarbons in surface seawater and in indigenous mussels (Mytilus galloprovincialis) from coastal areas of the Saronikos Gulf (Greece). Estuar Coast Shelf Sci 79(4):733–739
US EPA Method 61040 CFR Part 136, App. A-National Environment Methods, 2004.
Bruna F, Ceils R, Real M, Cornejo J (2012) Organo/LDH nanocomposites as an adsorbent of polycyclic aromatic hydrocarbons in water and soil–water systems. J Hazar Mate 225–226:74–80
Paolini J, Leandri C, Desjobert JM, Barboni T, Costa J (2008) Comparison of liquid–liquid extraction with headspace methods for the characterization of volatile fractions of commercial hydrolats from typically Mediterranean species. J Chromatogr A 1193(1–2):37–49
Zhao RS, Wang X, Yuan JP, Lin JM (2008) Investigation of feasibility of bamboo charcoal as solid-phase extraction adsorbent for the enrichment and determination of four phthalate esters in environmental water samples. J Chromatogr A 1183(1–2):15–20
Cortazar E, Zuloaga O, Sanz J, Raposo JC, Etxebarria N, Fernández LA (2002) MultiSimplex optimisation of the solid-phase microextraction-gas chromatographic–mass spectrometric determination of polycyclic aromatic hydrocarbons, polychlorinated biphenyls and phthalates from water samples. J Chromatogr A 978(1–2):165–175
Peñalver A, Pocurull E, Borrull F, Marcé RM (2001) Comparison of different fibers for the solid-phase microextraction of phthalate esters from water. J Chromatogr A 922(1–2):377–384
Psillakis E, Kalogerakis N (2003) Hollow-fibre liquid-phase microextraction of phthalate esters from water. J Chromatogr A 999(1–2):145–153
Zhao RS, Diao CP, Wang X, Jiang T, Yuan JP (2008) Rapid determination of amide herbicides in environmental water samples with dispersive liquid–liquid microextraction prior to gas chromatography–mass spectrometry. Anal Bioanal Chem 391(8):2915–2921
Cai YQ, Jiang GB, Liu JF, Zhou QX (2003) Multi-walled carbon nanotubes packed cartridge for the solid-phase extraction of several phthalate esters from water samples and their determination by high performance liquid chromatography. Anal Chim Acta 494(1–2):149–156
Shang XH, Yan XP (2007) Selective detection of trace lead in lead-free solder alloy by flow injection on-line solid-phase extraction using a macrocycle immobilized silica gel as sorbent coupled with hydride generation atomic fluorescence spectrometry. J Anal At Spectrom 22(10):1284–1289
Zhou QX, Mao JL, Xiao JP, Xie GH (2010) Determination of paraquat and diquat preconcentrated with N doped TiO2 nanotubes solid phase extraction cartridge prior to capillary electrophoresis. Anal Methods 2(8):1063–1068
Davi ML, Liboni M, Malfatto MG (1999) Multiresidue analysis of organic pollutants in water by SPE with a C8 and SDVB combined cartridge. Int J Environ Anal Chem 74(1–4):155–166
Holadova K, Hajslova J (1995) A comparison of different ways of sample preparation for the determination of phthalic acid esters in water and plant matrices. Int J Environ Anal Chem 59(1):43–47
Liu XY, Ji YS, Zhang YH, Zhang HX, Liu MC (2007) Oxidized multiwalled carbon nanotubes as a novel solid-phase microextraction fiber for determination of phenols in aqueous samples. J Chromatogr A 1165(1–2):10–17
Wang LJ, Wang LR, Feng YJ, Feng JT, Li DQ (2011) Highly efficient and selective infrared absorption material based on layered double hydroxides for use in agricultural plastic film. Appl Clay Sci 53(4):592–597
Lin YJ, Li DQ, Evans DG, Duan X (2005) Modulating effect of Mg–Al–CO3 layered double hydroxides on the thermal stability of PVC resin. Polym Degrad Stab 88(2):286–293
Cui GJ, Evans DG, Li DQ (2010) Synthesis and UV absorption properties of 5′5′-thiodisalicylic acid intercalated Zn–Al layered double hydroxides. Polym Degrad Stab 95(10):2082–2087
Busca G, Costantino U, Montanari T, Ramis G, Resini C, Sisani M (2010) Nickel versus cobalt catalysts for hydrogen production by ethanol steam reforming: Ni–Co–Zn–Al catalysts from hydrotalcite-like precursors. Int J Hydrogen Energy 35(11):5356–5366
Behrens M, Kasatkin I, Kühl S, Weinberg G (2010) Phase-pure Cu, Zn, Al hydrotalcite-like materials as precursors for copper rich Cu/ZnO/Al2O3 catalysts. Chem Mater 22(2):386–397
Zhang GB, Ding P, Zhang M, Qu BJ (2007) Synergistic effects of layered double hydroxide with hyperfine magnesium hydroxide in halogen-free flame retardant EVA/HFMH/LDH nanocomposites. Polym Degrad Stab 92(9):1715–1720
Zhou JB, Yang SL, Yu JG, Shu Z (2011) Novel hollow microspheres of hierarchical zinc–aluminum layered double hydroxides and their enhanced adsorption capacity for phosphate in water. J Hazard Mater 192(3):1114–1121
Li BX, He J, Evans DG, Duan X (2004) Inorganic layered double hydroxides as a drug delivery system—intercalation and in vitro release of fenbufen. Appl Clay Sci 27(3–4):199–207
Shan D, Yao WJ, Xue HG (2007) Electrochemical study of ferrocenemethanol-modified layered double hydroxides composite matrix: application to glucose amperometric biosensor. Biosens Bioelectron 23(3):432–437
Ulibarri MA, Pavlovic I, Hermosfn MC, Cornejo J (1995) Hydrotalcite-like compounds as potential sorbents of phenols from water. Appl Clay Sci 10(1–2):131–145
Chaara D, Pavlovic I, Bruna F, Ulibarri MA, Draoui K, Barriga C (2010) Removal of nitrophenolpesticides from aqueous solutions by layered double hydroxides and their calcined products. Appl Clay Sci 50(3):292–298
Chuang YH, Liu CH, Tzou YM, Chang JS, Chiang PN, Wang MK (2010) Comparison and characterization of chemical surfactants and bio-surfactants intercalated with layered double hydroxides (LDHs) for removing naphthalene from contaminated aqueous solutions. Colloids Surf, A Physicochem Eng Asp 366(1–3):170–177
Gao ZY, Du B, Zhang GY, Gao Y, Li ZJ, Zhang H, Duan X (2011) Adsorption of pentachlorophenol from aqueous solution on dodecylbenzenesulfonate modified nickel − titanium layered double hydroxide nanocomposites. Ind Eng Chem Res 50(9):5334–5345
Zhang H, Wen X, Wang YX (2007) Synthesis and characterization of sulfate and dodecylbenzenesulfonate intercalated zinc–iron layered double hydroxides by one-step coprecipitation route. J Solid State Chem 180(5):1636–1647
Zaghouane-Boudiaf H, Boutahala M, Tiar C, Arab L, Garin F (2011) Treatment of 2,4,5-trichlorophenol by MgAl-SDBS organo-layered double hydroxides: kinetic and equilibrium studies. Chem Eng J 173(1):36–41
Bouraada M, Lafjah M, Ouali MS, Menorval LC (2008) Basic dye removal from aqueous solutions by dodecylsulfate- and dodecyl benzene sulfonate-intercalated hydrotalcite. J Hazard Mater 153(3):911–918
Chen BL, Zhu LZ (2001) Partition of polycyclic aromatic hydrocarbons on organobentonites. J Environ Sci-CHINA 13(2):129–136
Li K, Li HF, Liu LB, Hashi Y, Maeda T, Lin JM (2007) Solid-phase extraction with C30 bonded silica for analysis of polycyclic aromatic hydrocarbons in airborne particulate matters by gas chromatography–mass spectrometry. J Chromatogr A 1154(1–2):74–80
King AJ, Readman JW, Zhou JL (2004) Determination of polycyclic aromatic hydrocarbons in water by solid-phase microextraction–gas chromatography–mass spectrometry. Anal Chim Acta 523(2):259–267
Acknowledgments
This work is jointly supported by the National Natural Science Foundation of China (21007035), Scientific and Technological Developing Project of Shandong Province (2011SJGZ03), Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (20101j0061), and Basic Foundation of Shandong Academy of Sciences (201047). We thank Lorna and Prof. Tom Welton (Imperial College, UK) for English improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, YL., Zhou, JB., Zhao, RS. et al. Using Zn/Al layered double hydroxide as a novel solid-phase extraction adsorbent to extract polycyclic aromatic hydrocarbons at trace levels in water samples prior to the determination of gas chromatography–mass spectrometry. Anal Bioanal Chem 404, 1603–1610 (2012). https://doi.org/10.1007/s00216-012-6219-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6219-9