Abstract
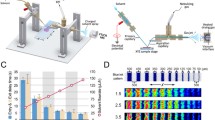
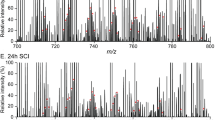
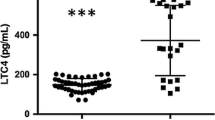
We determined quantitative and qualitative alterations in lipids during the occurrence and progression of spinal cord injury (SCI) in rats to identify potential clinical indicators of SCI pathology. Imaging mass spectrometry (IMS) was used to visualize twelve molecular species of phosphatidylcholine (PC) on thin slices of spinal cord with SCI. In addition, twelve species of phospholipids and five species of prostaglandins (PGs) were quantified by liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS) of lipid extracts from control/injured spinal cords. Unique distribution patterns were observed for phospholipids with different fatty acid compositions, and distinct dynamic changes were seen in both their amounts and their distributions in tissue as tissue damage resulting from SCI progressed. In particular, PCs containing docosahexaenoic acid localized to the large nucleus in the anterior horn region at one day post-SCI and rapidly decreased thereafter. In contrast, PCs containing arachidonic acid (AA-PCs) were normally found in the posterior horn region and were intensely and temporarily elevated one week after SCI. Lysophosphatidylcholines (LPCs) also increased at the same SCI stage and in regions with elevated AA-PCs, indicating the release of AA and the production of PGs. Moreover, LC-ESI-MS/MS analysis of lipid extracts from the spinal cord tissue at the impact site demonstrated a peak in PGE2 that reflected the elevation/reduction pattern of AA-PCs and LPC. Although further investigation is required, we suggest that invasive immune cells that penetrated from the impaired blood–brain barrier at 1–2 weeks post-SCI may have produced LPCs, released AA from AA-PCs, and produced PGs in SCI tissue at sites enriched in AA-PCs/LPC.







Similar content being viewed by others
References
Piomelli D, Astarita G, Rapaka R (2007) A neuroscientist's guide to lipidomics. Nat Rev Neurosci 8:743–754
Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R (2006) Molecular anatomy of a trafficking organelle. Cell 127:831–846
Rohrbough J, Broadie K (2005) Lipid regulation of the synaptic vesicle cycle. Nat Rev Neurosci 6:139–150
Jacobson K, Mouritsen OG, Anderson RG (2007) Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol 9:7–14
Allen JA, Halverson-Tamboli RA, Rasenick MM (2007) Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8:128–140
Williams JH, Errington ML, Lynch MA, Bliss TV (1989) Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature 341:739–742
Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA 99:10819–10824
Hermann GE, Rogers RC, Bresnahan JC, Beattie MS (2001) Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis 8(4):590–599
Buczynski MW, Svensson CI, Dumlao DS, Fitzsimmons BL, Shim JH, Scherbart TJ, Jacobsen FE, Hua XY, Yaksh TL, Dennis EA (2010) Inflammatory hyperalgesia induces essential bioactive lipid production in the spinal cord. J Neurochem 114(4):981–993
Ma L, Nagai J, Ueda H (2010) Microglial activation mediates de novo lysophosphatidic acid production in a model of neuropathic pain. J Neurochem 115(3):643–653
Nagai J, Ueda H (2011) Pre-emptive morphine treatment abolishes nerve injury-induced lysophospholipid synthesis in mass spectrometrical analysis. J Neurochem 118(2):256–265
Buczynski MW, Svensson CI, Dumlao DS, Fitzsimmons BL, Shim JH, Scherbart TJ, Jacobsen FE, Hua XY, Yaksh TL, Dennis EA (2010) Inflammatory hyperalgesia induces essential bioactive lipid production in the spinal cord. J Neurochem 114(4):981–993
Girod M, Shi Y, Cheng JX, Cooks RG (2011) Mapping lipid alterations in traumatically injured rat spinal cord by desorption electrospray ionization imaging mass spectrometry. Anal Chem 83:207–215
Sugiura Y, Setou M (2010) Imaging mass spectrometry for visualization of drug and endogenous metabolite distribution: toward in situ pharmacometabolomes. J Neuroimmune Pharmacol 5:31–43
Garrett TJ, Yost RA (2006) Analysis of intact tissue by intermediate-pressure MALDI on a linear ion trap mass spectrometer. Anal Chem 78:2465–2469
Jackson SN, Woods AS (2009) Direct profiling of tissue lipids by MALDI-TOFMS. J Chromatogr B 877:2822–2829
Cornett DS, Reyzer ML, Chaurand P, Caprioli RM (2007) MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat Methods 4:828–833
Sugiura Y, Konishi Y, Zaima N, Kajihara S, Nakanishi H, Taguchi R, Setou M (2009) Visualization of the cell-selective distribution of PUFA-containing phosphatidylcholines in mouse brain by imaging mass spectrometry. J Lipid Res 50:1776–1788
Murphy RC, Hankin JA, Barkley RM (2009) Imaging of lipid species by MALDI mass spectrometry. J Lipid Res 50(Suppl):S317–S322
Touboul D, Brunelle A, Halgand F, De La Porte S, Laprévote O (2005) Lipid imaging by gold cluster time-of-flight secondary ion mass spectrometry: application to Duchenne muscular dystrophy. J Lipid Res 46:1388–1395
Sugiura Y, Taguchi R, Setou M (2011) Visualization of spatiotemporal energy dynamics of hippocampal neurons by mass spectrometry during a kainate-induced seizure. PLoS One 6(3):e17952
Sugiura Y, Shimma S, Konishi Y, Yamada MK, Setou M (2008) Imaging mass spectrometry technology and application on ganglioside study; visualization of age-dependent accumulation of C20-ganglioside molecular species in the mouse hippocampus. PLoS One 3:e3232
Delvolve AM, Colsch B, Woods AS (2011) Highlighting anatomical sub-structures in rat brain tissue using lipid imaging. Anal Meth 3:1729–1736
Setou M (ed)(2010) Imaging mass spectrometry (Protocols for Mass Microscopy). Springer, Berlin
Burnum KE, Cornett DS, Puolitaival SM, Milne SB, Myers DS, Tranguch S, Brown HA, Dey SK, Caprioli RM (2009) Spatial and temporal alterations of phospholipids determined by mass spectrometry during mouse embryo implantation. J Lipid Res 50:2290–2298
Matsumoto J, Sugiura Y, Yuki D, Hayasaka T, Goto-Inoue N, Zaima N, Kunii Y, Wada A, Yang Q, Nishiura K, Akatsu H, Hori A, Hashizume Y, Yamamoto T, Ikemoto K, Setou M, Niwa S (2011) Abnormal phospholipids distribution in the prefrontal cortex from a patient with schizophrenia revealed by matrix-assisted laser desorption/ionization imaging mass spectrometry. Anal Bioanal Chem 400:1933–1943
Beck G, Sugiura Y, Shinzawa K, Kato S, Setou M, Tsujimoto Y, Sakoda S, Sumi-Akamaru H (2011) Neuroaxonal dystrophy in calcium-independent phospholipase A2beta deficiency results from insufficient remodeling and degeneration of mitochondrial and presynaptic membranes. J Neurosci 31:11411–11420
Kita Y, Takahashi T, Uozumi N, Shimizu T (2005) A multiplex quantitation method for eicosanoids and platelet-activating factor using column-switching reversed-phase liquid chromatography-tandem mass spectrometry. Anal Biochem 342:134–143
Puolitaival SM, Burnum KE, Cornett DS, Caprioli RM (2008) Solvent-free matrix dry-coating for MALDI imaging of phospholipids. J Am Soc Mass Spectrom 19:882–886
Hankin JA, Barkley RM, Murphy RC (2007) Sublimation as a method of matrix application for mass spectrometric imaging. J Am Soc Mass Spectrom 18:1646–1652
Zhao P, Waxman SG, Hains BC (2007) Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci 27(9):2357–2368
Redensek A, Rathore KI, Berard JL, López-Vales R, Swayne LA, Bennett SA, Mohri I, Taniike M, Urade Y, David S (2011) Expression and detrimental role of hematopoietic prostaglandin D synthase in spinal cord contusion injury. Glia 59(4):603–614
Zhang J, Fujii S, Wu Z, Hashioka S, Tanaka Y, Shiratsuchi A, Nakanishi Y, Nakanishi H (2006) Involvement of COX-1 and up-regulated prostaglandin E synthases in phosphatidylserine liposome-induced prostaglandin E2 production by microglia. J Neuroimmunol 172:112–120
Yoshikawa K, Kita Y, Kishimoto K, Shimizu T (2006) Profiling of eicosanoid production in the rat hippocampus during kainic acid-induced seizure: dual phase regulation and differential involvement of COX-1 and COX-2. J Biol Chem 281:14663–14669
Minghetti L, Polazzi E, Nicolini A, Levi G (1998) Opposite regulation of prostaglandin E2 synthesis by transforming growth factor-beta1 and interleukin 10 in activated microglial cultures. J Neuroimmunol 82:31–39
Hoozemans JJ, Veerhuis R, Janssen I, van Elk EJ, Rozemuller AJ, Eikelenboom P (2002) The role of cyclo-oxygenase 1 and 2 activity in prostaglandin E(2) secretion by cultured human adult microglia: implications for Alzheimer’s disease. Brain Res 951:218–226
Ajmone-Cat MA, Nicolini A, Minghetti L (2003) Prolonged exposure of microglia to lipopolysaccharide modifies the intracellular signaling pathways and selectively promotes prostaglandin E2 synthesis. J Neurochem 87:1193–1203
Ikeda-Matsuo Y, Ikegaya Y, Matsuki N, Uematsu S, Akira S, Sasaki Y (2005) Microglia-specific expression of microsomal prostaglandin E2 synthase-1 contributes to lipopolysaccharide-induced prostaglandin E2 production. J Neurochem 94:1546–1558
Acknowledgments
This study was supported by research grants for PRESTO for Y.S. and SENTAN from the JST, and a Grant-In-Aid for Young Scientists (S) from the JSPS for M.S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mitsuru Hanada and Yuki Sugiura contributed equally to this work.
Published in the special paper collection Biomedical Mass Spectrometry with guest editors Toyofumi Nakanishi and Mitsutoshi Setou.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 200 kb)
Rights and permissions
About this article
Cite this article
Hanada, M., Sugiura, Y., Shinjo, R. et al. Spatiotemporal alteration of phospholipids and prostaglandins in a rat model of spinal cord injury. Anal Bioanal Chem 403, 1873–1884 (2012). https://doi.org/10.1007/s00216-012-5900-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-5900-3