Abstract
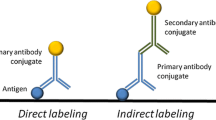
There are several analytical techniques currently in use in conservation science to identify proteins in artworks. However, as is often the case, the determination of the exact location of a protein in a complex layer structure is challenging due to difficulty in separating layers. Localization of the protein in a cross-section has been demonstrated through attenuated total reflectance-Fourier transform infrared mapping and imaging as well as chemiluminescent and fluorescent-labeled antibodies; however, these techniques either require expensive instrumental setups or produce results that can be challenging to interpret. This paper will present research using surface-enhanced Raman scattering (SERS) nanotags complexed to secondary antibodies in conjunction with primary antibodies for the localization of ovalbumin, collagen, and casein in cross-sections from replicas and artworks containing avian egg, animal glue, or casein binders. The advantages of this technique over the others are (1) the detection method is a Raman microscope, equipment found in several museum laboratories; (2) the distinctive SERS signal from the nanotag increases the detection limit of the protein and decreases the interference from other colorants present in the cross-section layers; and finally, (3) the large (120 nm) SERS-labeled antibodies do not appear to penetrate into the cross-section, eliminating the risk of spurious signal and misidentification. Any agglomerations due to surface texture are clearly visible under normal illumination and can be avoided easily during analysis or removed with a light polish. This technique not only allows protein localization in multilayered samples while preserving the stratigraphic information but also retains the protein specificity of the antibody approach.












Similar content being viewed by others
Notes
ATR-FTIR analysis performed by Adriana Rizzo, Associate Scientist, Department of Scientific Research, The Metropolitan Museum of Art, New York, USA.
FTIR and GC/MS analysis performed by Adriana Rizzo, Associate Scientist, Department of Scientific Research, The Metropolitan Museum of Art, New York, USA.
KPL, Kirkegaard & Perry Laboratories, Inc. online catalogue, http://www.kpl.com/catalog/categories.cfm?Catalog_ID=17&Category_ID=482
References
Spring M, Ricci C, Peggie DA, Kazarian SG (2008) ATR-FTIR imaging for the analysis of organic materials in paint cross sections: case studies on paint samples from the National Gallery London. Anal Bioanal Chem 392:37–45
Rizzo A (2008) Progress in the application of ATR-FTIR microscopy to the study of multi-layered cross-sections from works of art. Anal Bioanal Chem 392:47–55
Mazzeo R, Prati S, Quaranta M, Joseph E, Kendix E, Galeotti M (2008) Attenuated total reflection micro FTIR characterization of pigment–binder interaction in reconstructed paint films. Anal Bioanal Chem 392:65–76
Fonjaudran CMd, Nevin A, Piqué F, Cather S (2008) Stratigraphic analysis of organic materials in wall painting samples using micro-FTIR attenuated total reflectance and a novel sample preparation technique. Anal Bioanal Chem 392:77–86
Hodgins G (1999) Investigating methods of identifying pre-renaissance artists’ paints and glues. University of Oxford, Oxford
Heginbotham A, Millay V, Quick M (2004) The use of immunofluorescence microscopy (IFM) and enzyme-linked immunosorbent assay (ELISA) as complementary techniques for protein identification in artists’ materials. J Am Inst Conserv 45:89–106
Mazurek J, Heginbotham A, Schilling M, Chiari G (2008) Antibody assay to characterize binding media in paint. ICOM Comm Conserv 2:678–685
Klausmeyer PA, Albertson RP, Schmidt MR, Woodland RT, Blewett M (2009) Analysis and treatment of a painting by Kees van Dongen: FTIR and ELISA as complementary techniques in the analysis of art materials. e-Preservation. Science 6:151–162
Arslanoglu J, Schultz J, Loike J, Petersen K (2010) Immunology and art: using antibody-based techniques to identify proteins and gums in artworks. J Biosci 35:3–10
Schultz J, Arslanoglu J, Tavzes C, Petersen K (2009) Immunological techniques: a different approach to the analysis of proteins in cultural heritage. Part I: the basics explained. Z Kunsttechnol Konserv 23:129–139
Schultz J (2006) Immunolgische Methoden zur Analytik tierischer Bindemittel–Möglichkeiten und Grenzen. HAWK University of Applied Sciences and Arts of Hildesheim, Holzminden
Dolci LS, Sciutto G, Guardigli M, Rizzoli M, Prati S, Mazzeo R, Roda A (2008) Ultrasensitive chemiluminescent immunochemical identification and localization of protein components in painting cross-sections by microscope low-light imaging. Anal Bioanal Chem 392:29–35
Cartechini L, Vagnini M, Palmieri M, Pitzurra L, Mello T, Mazurek J, Chiari aG (2010) Immunodetection of proteins in ancient paint media. Acc Chem Res 43:867–876
Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie aS (2005) In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotechnol 16:63–72
Frasco MF, Chaniotakis N (2010) Bioconjugated quantum dots as fluorescent probes for bioanalytical applications. Anal Bioanal Chem 396:229–240
Metiu H, Das P (1984) The electromagnetic theory of surface enhanced spectroscopy. Annu Rev Phys Chem 37:507–536
Schatz GC (1984) Theoretical studies of surface enhanced Raman scattering. Acc Chem Res 17:370–376
Moskovits M (1985) Surface-enhanced spectroscopy. Rev Mod Phys 57:783–826
Creighton JA (1988) Selection rules for surface-enhanced Raman spectroscopy. In: Clark RJH, Wester RE (eds) Spectroscopy of surfaces. Wiley, New York, pp 39–109
Xu H, Aizpurua J, Käll M, Apell PA (2000) Electromagnetic contributions to single-molecule sensitivity in surface-enhanced Raman scattering. Phys Rev E 62:4318–4324
Schatz GC, Van Duyne RP (2002) Electromagnetic mechanism of surface-enhanced spectroscopy. In: Chalmers JM, Griffiths PR (eds) Handbook of vibrational spectroscopy. Wiley, New York, pp 759–774
Canamares MV, Garcia-Ramos JV, Domingo C, Sanchez-Cortes S (2004) Surface-enhanced Raman scattering study of the adsorption of the anthraquinone pigment alizarin on Ag nanoparticles. J Raman Spectrosc 35:921–927
Centeno SA, Shamir J (2008) Surface enhanced Raman scattering (SERS) and FTIR characterization of the sepia melanin pigment used in works of art. J Mol Struct 873:149–159
Leona M (2009) Microanalysis of organic pigments and glazes in polychrome works of art by surface-enhanced resonance Raman scattering. PNAS 106:14757–14762
Kim J-H, Kim J-S, Choi H, Lee S-M, Jun B-H, Yu K-N, Kuk E, Kim Y-K, Jeong DH, Cho M-H, Lee Y-S (2006) Nanoparticle probes with surface enhanced Raman spectroscopic tags for cellular cancer targeting. Anal Chem 78:6967–6973
Sun L, Sung K-B, Dentinger C, Lutz B, Nguyen L, Zhang J, Qin H, Yamakawa M, Cao M, Lu Y, Chmura A, Zhu J, Su X, Berlin AA, Chan S, Knudsen B (2007) Composite organic–inorganic nanoparticles as Raman labels for tissue analysis. Nano Lett 7:351–356
Yu KN, Lee S-M, Han JY, Park H, Woo M-A, Noh MS, Hwang S-K, Kwon J-T, Jin H, Kim Y-K, Hergenrother PJ, Jeong DH, Lee Y-S, Cho M-H (2007) Multiplex targeting, tracking, and imaging of apoptosis by fluorescent surface enhanced Raman spectroscopic dots. Bioconjug Chem 18:1155–1162
Chen J-W, Lei Y, Liu X-J, Jiang J-H, Shen G-L, Yu R-Q (2008) Immunoassay using surface-enhanced Raman scattering based on aggregation of reporter-labeled immunogold nanoparticles. Anal Bioanal Chem 392:187–193
Zhang X, Yin H, Cooper JM, Haswell SJ (2008) Characterization of cellular chemical dynamics using combined microfluidic and Raman techniques. Anal Bioanal Chem 390:833–840
Giesfeldt KS, Connatser RM, De Jesus MA, Dutta P, Sepaniak MJ (2005) Gold-polymer nanocomposites: studies of their optical properties and their potential as SERS substrates. J Raman Spectrosc 36:1134–1142
Doering WE, Piotti ME, Natan MJ, Freeman RG (2007) SERS as a foundation for nanoscale, optically detected biological labels. Adv Mater 19:3100–3108
Sha MY, Xu H, Natan MJ, Cromer R (2008) Surface-enhanced Raman scattering tags for rapid and homogeneous detection of circulating tumor cells in the presence of human whole blood. J Am Chem Soc 130:17214–17215
Zavaleta CL, Smith BR, Walton I, Doering W, Davis G, Shojaei B, Natan MJ, Gambhir SS (2009) Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. PNAS 106:13511–13516
Mulvaney SP, He L, Natan MJ, Keating CD (2002) Three-layer substrates for surface-enhanced Raman scattering: preparation and preliminary evaluation. J Raman Spectrosc 34:163–171
Fleischmann M, Hendra PJ, McQuillan AJ (1974) Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett 26:63–166
Keren S, Zavaleta C, Cheng Z, Zerda Adl, Gheysens O, Gambhir SS (2008) Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc Natl Acad Sci USA 105:5844–5849
Nadolny J (2006) All that’s burnished isn’t bole’: reflections on Medieval water gilding, part 1. Early Medieval to 1300. In: Nadolny J (ed) Medieval painting in Northern Europe: techniques, analysis, art history. Archetype Books, London, pp 150–152
Hale C, Arslanoglu J, Centeno SA (2010) Granacci in The Metropolitan Museum of Art: aspects of evolving workshop practice. The National Gallery Technical Bulletin 30th Anniversary Conference; Studying Old Master Paintings, Technology and Practice, London (in press)
Boon JJ, Peulve SL, Van den Brink OE, Duursma MC, Rainford D (1996) Molecular aspects of mobile and stationary phases in ageing tempera and oil paint films. In: Bakkenist T, Hoppenbrouwers R, Dubois H (eds) Early Italian painting techniques and analysis. Limburg Conservation Institute, Maastricht, pp 35–56
Castelnuovo-Tedesco L, Soultanian J (2010) Italian Medieval sculpture in the Metropolitan Museum and the Cloisters. Metropolitan Museum of Art, New York
Acknowledgments
The authors gratefully acknowledge Julia Schultz (Hochschule für Angewandte Wissenschaft und Kunst (HAWK) Fachhochschule, Hildesheim, Germany), for her generosity in allowing the reproduction of her data and the use of her methodologies in this paper and her great contribution to the immunological project at the Met during her Fellowship (2007–2009), as well as her doctoral supervisor, Dr. Karin Peterson (HAWK). The authors also thank our colleagues from the Metropolitan Museum of Art: Adriana Rizzo and Silvia Centeno, from The Department of Scientific Research, for providing samples, analytical results, and invaluable discussions; Jack Soultanian, Objects Conservator, for initiating the study of the St. John sculpture (MMA 25.120.215); and Charlotte Hale, Paintings Conservator, for launching the study of The Crucifixion (MMA 2006.409) and Scenes from the Life of St. John the Baptist (1970.134.1). Finally, we thank Dina Georgas (Barnard College, New York) for her invaluable assistance with this paper and the immunological project at the Met.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Analytical Chemistry for Cultural Heritage with Guest Editors Rocco Mazzeo, Silvia Prati, and Aldo Roda.
Rights and permissions
About this article
Cite this article
Arslanoglu, J., Zaleski, S. & Loike, J. An improved method of protein localization in artworks through SERS nanotag-complexed antibodies. Anal Bioanal Chem 399, 2997–3010 (2011). https://doi.org/10.1007/s00216-010-4378-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4378-0