Abstract
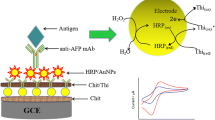
A novel experimental methodology based on a Prussian blue (PB) and gold nanoparticles (AuNPs) modified carbon ionic liquid electrode (CILE) was developed for use in a label-free amperometric immunosensor for the sensitive detection of human immunoglobulin G (HIgG) as a model protein. The CILE was fabricated by using the ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate as binder. Controllable electrodeposition of PB on the surface of the CILE and coating with 3-aminopropyl triethylene silane (APS) formed a film with high electronic catalytic activity and large surface area for the assembly of AuNPs and further immobilization of HIgG antibody. The electrochemistry of the formed nanocomposite biofilm was investigated by electrochemical techniques including cyclic voltammetry, differential pulse voltammetry, and electrochemical impedance spectroscopy. The HIgG concentration was measured through the decrease of amperometric responses in the corresponding specific binding of antigen and antibody. The decreased differential pulse voltammetric values were proportional to the HIgG concentration in two ranges, 0.05–1.25 ng mL−1 and 1.25–40 ng mL−1, with a detection limit of 0.001 ng mL−1 (S/N = 3). This electrochemical immunoassay combined the specificity of the immunological reaction with the sensitivity of the AuNPs, ionic liquid, and PB amplified electrochemical detection and would therefore be valuable for clinical immunoassays.

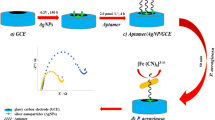
Cyclic voltammograms of the immunosensor in pH 7.0 phosphate-buffered solution containing 0.1 M KCl at the different scan rate of (from inner to outer) 50, 100, 150, 200, 250, 300, 350, 400, 450, 500, 550, 600, 650, 700, 750, and 800 mVs−1. The inset shows the linear relationship between the peak currents and the square root of scan rate.






Similar content being viewed by others
References
Duffy MJ, Van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, Nilsson O, Sturgeon C, Topolcan O (2003) Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer 39:718–727
Hernadez L, Espasa A, Fenandez C, Candela V, Martin C, Romero S (2000) CEA and CA 549 in serum and pleural fluid of patients with pleural effusion. Lung Cancer 36:83–89
Morozov VN, Morozova TY (2003) Electrophoresis-assisted active immunoassay. Anal Chem 75:6813–6819
Ehrhart J, Bennetau B, Renaud L, Madrange J, Thomas L, Morisot J, Brosseau A, Tran P (2008) A new immunosensor for breast cancer cell detection using antibody-coated long alkylsilane self-assembled monolayers in a parallel plate flow chamber. Biosens Bioelectron 24:467–474
Kim N, Kim D, Cho Y, Moon D, Kim W (2008) Carp vitellogenin detection by an optical waveguide lightmode spectroscopy biosensor. Biosens Bioelectron 24:391–396
Wasowicz M, Viswanathan S, Dvornyk A, Grzelak K, Kludkiewicz B, Radecka H (2008) Development of an oligo(ethylene glycol)-based SPR immunosensor for TNT detection. Biosens Bioelectron 24:191–197
Yang Z, Fu Z, Yan F, Liu H, Ju H (2008) A chemiluminescent immunosensor based on antibody immobilized carboxylic resin beads coupled with micro-bubble accelerated immunoreaction for fast flow-injection immunoassay. Biosens Bioelectron 24:35–40
Campas M, Marty J (2007) Enzyme sensor for the electrochemical detection of the marine toxin okadaic acid. Anal Chim Acta 605:87–93
Pohanka M, Skladal P (2008) Electrochemical biosensors - principles and applications. J Appl Biomed 6:57–64
Li J, Tan SN, Ge H (1996) Silica sol-gel immobilized amperometric biosensor for hydrogen peroxide. Anal Chim Acta 335:137–138
Razumiene J, Meškys R, Gurevièiene V, Laurinavièius V, Reshetova MD, Ryabov AD (2000) 4-Ferrocenylphenol as an electron transfer mediator in PQQ-dependent alcohol and glucose dehydrogenase-catalyzed reactions. Electrochem Commun 2:307–311
Yu H, Yan F, Dai Z, Ju H (2004) A disposable amperometric immunosensor for α-1-fetoprotein based on enzyme-labeled antibody/chitosan-membrane-modified screen-printed carbon electrode. Anal Biochem 331:98–105
Lei CX, Wu J, Wang H, Shen GL, Yu RQ (2004) A new electrochemical immunoassay strategy for detection of transferrin based on electrostatic interaction of natural polymers. Talanta 63:469–474
Li N, Yuan R, Chai Y, Chen S, An H, Li W (2007) New antibody immobilization strategy based on gold nanoparticles and Azure I/multi-walled carbon nanotube composite membranes for an amperometric enzyme immunosensor. J Phys Chem C 111:8443–8450
Fiorito PA, Goncales VR, Ponzio EA (2005) Synthesis, characterization and immobilization of Prussian blue nanoparticles: a potential tool for biosensing devices. Chem Commun 5:366–368
Oliveira MF, Mortimer RJ, Stradiotto NR (2000) Voltammetric determination of persulfate anions using an electrode modified with a Prussian blue film. Microchem J 64:155–160
Ricci F, Moscone D, Tuta CS, Palleschi G, Amine A, Poscia A, Valgimigli F, Messeri D (2005) Novel planar glucose biosensors for continuous monitoring use. Biosens Bioelectron 20:1993–2000
Zhao G, Feng JJ, Zhang QL, Li SP, Chen HY (2005) Synthesis and characterization of Prussian blue modified magnetite nanoparticles and its application to the electrocatalytic reduction of H2O2. Chem Mater 17:3154–3159
Zhou P, Xue D, Luo H, Chen X (2002) Structure and magnetic properties of highly ordered Prussian blue nanowire arrays. Nano Lett 2:845–847
Mattos IL, Lukachova LV, Gorton L, Laurell T, Karyakin AA (2001) Evaluation of glucose biosensors based on Prussian Blue and lyophilised, crystalline and cross-linked glucose oxidases (CLEC®). Talanta 54:963–974
Karyakin AA, Puganova EA, Budashov IA, Kurochkin IN, Karyakina EE, Levchenko VA, Matveyenko VN, Varfolomeyev SD (2004) Prussian blue based nanoelectrode arrays for H2O2 detection. Anal Chem 76:474–478
Garjonyte R, Malinauskas A (1999) Operational stability of amperometric hydrogen peroxide sensors, based on ferrous and copper hexacyanoferrates. Sens Actuators B 56:93–97
Liu ZM, Yang Y, Wang H, Liu YL, Shen GL, Yu RQ (2005) A hydrogen peroxide biosensor based on nano-Au/PAMAM dendrimer/cystamine modified gold electrode. Sens Actuators B 106:394–400
Xiao Y, Patolsky F, Katz E, Hainfeld JF, Willner I (2003) “Plugging into enzymes”: nanowiring of redox enzymes by a gold nanoparticle. Science 299:1877–1881
Parak WJ, Pellegrino T, Micheel CM, Gerion D, Williams SC, Alivisatos AP (2003) Conformation of oligonucleotides attached to gold nanocrystals probed by gel-electrophoresis. Nano Lett 3:33–36
Gole A, Dash C, Soman C, Sainkar SR, Rao M, Sastry M (2001) On the preparation, characterization, and enzymatic activity of fungal protease-gold colloid bioconjugates. Bioconjug Chem 12:684–690
Chen SH, Yuan R, Chai YQ, Xu Y, Min LG, Li N (2008) A new antibody immobilization technique based on organic polymers protected Prussian blue nanoparticles and gold colloidal nanoparticles for amperometric immunosensors. Sens Actuators B 135:236–244
Abbaspour A, Mehrgardi MA (2004) Electrocatalytic oxidation of guanine and DNA on a carbon paste electrode modified by cobalt hexacyanoferrate films. Anal Chem 76:5690–5696
Lawrence NS, Deo RP, Wang J (2004) Biocatalytic carbon paste sensors based on a mediator pasting liquid. Anal Chem 76:3735–3739
Li CM, Zang JF, Zhan DP, Chen W, Sun CQ, Teo AL, Chua YT, Lee VS, Moochhala SM (2006) Electrochemical detection of nitric oxide on a SWCNT/RTIL composite gel microelectrode. Electroanalysis 18:713–718
Sun W, Yang MX, Gao RF, Jiao K (2007) Electrochemical determination of ascorbic acid in room temperature ionic liquid Bpp f6 modified carbon paste electrode. Electroanalysis 19:1597–1602
Yan QP, Zhao FQ, Li GZ, Zeng BZ (2006) Voltammetric determination of uric acid with a glassy carbon electrode coated by paste of multiwalled carbon nanotubes and ionic liquid. Electroanalysis 18:1075–1080
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22
Yoshio M, Mukai T, Ohno H, Kato T (2004) One-dimensional ion transport in self-organized columnar ionic liquids. J Am Chem Soc 126:994–995
Chen H, Jiang JH, Huang Y, Deng T, Li JS, Shen GL, Yu RQ (2006) An electrochemical impedance immunosensor with signal amplification based on Au-colloid labeled antibody complex. Sens Actuators B 117:211–218
Mao X, Jiang JH, Luo Y, Shen GL, Yu RQ (2007) Copper-enhanced gold nanoparticle tags for electrochemical stripping detection of human IgG. Talanta 73:420–424
Wang ZJ, Yang YH, Li JS, Gong JL, Shen GL, Yu RQ (2006) Organic–inorganic matrix for electrochemical immunoassay: detection of human IgG based on ZnO/chitosan composite. Talanta 69:686–690
Zhong ZY, Li MX, Xiang DB, Dai N, Qing Y, Wang D, Tang DP (2009) Signal amplification of electrochemical immunosensor for the detection of human serum IgG using double-codified nanosilica particles as labels. Biosens Bioelectron 24:2246–2249
Chen ZP, Peng ZF, Zhang P, Jin XF, Jiang JH, Zhang XB, Shen GL, Yu RQ (2007) A sensitive immunosensor using colloidal gold as electrochemical label. Talanta 72:1800–1804
Zhang SB, Zheng F, Wu ZS, Shen GL, Yu RQ (2008) Highly sensitive electrochemical detection of immunospecies based on combination of Fc label and PPD film/gold nanoparticle amplification. Biosens Bioelectron 24:129–135
Zhang LY, Liu Y, Chen T (2008) A mediatorless and label-free amperometric immunosensor for detection of h-IgG. Int J Biol Macromol 43:165–169
Luo Y, Mao X, Peng ZF, Jiang JH, Shen GL, Yu RQ (2008) A new strategy for electrochemical immunoassay based on enzymatic silver deposition on agarose beads. Talanta 74:1642–1648
Wang SP, Wu ZS, Qu FL, Zhang SB, Shen GL, Yu RQ (2008) A novel electrochemical immunosensor based on ordered Au nano-prickle clusters. Biosens Bioelectron 24:1020–1026
Acknowledgments
This work was supported by National Natural Science Foundation of China (20805040, 20635020), Excellent Youth Foundation of He’nan Scientific Committee (104100510020), China Postdoctoral Science Foundation funded project (200902508, 20080430163), Jiangsu Planned Projects for Postdoctoral Research Funds (0801041B), and sponsored by Program for Science & Technology Innovation Talents in Universities of Henan Province (2010HASTIT025).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 493 kb)
Rights and permissions
About this article
Cite this article
Huang, KJ., Niu, DJ., Sun, JY. et al. Label-free amperometric immunobiosensor based on a gold colloid and Prussian blue nanocomposite film modified carbon ionic liquid electrode. Anal Bioanal Chem 397, 3553–3561 (2010). https://doi.org/10.1007/s00216-010-3868-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3868-4