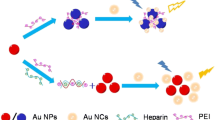
Abstract
In 2008, heparin contaminated with oversulfated chondroitin sulfate (OSCS) penetrated the worldwide market and was associated with severe adverse effects. Feasible and reliable methods to test heparin for adulteration are needed. The objective was to develop a simple approach based on a microplate assay for quantification of heparin and sulfated glycans using the fluorescent heparin sensor polymer-H (polymer-H assay). However, both heparin and OSCS concentration-dependently increase the fluorescence intensity (FI) of polymer-H, so that OSCS in heparin cannot be detected. The idea was a two-step procedure including, first, separation of heparin by degradation with heparinase I, and then measurement of the remaining OSCS. To achieve complete heparin (unfractionated heparin (UFH), enoxaparin) degradation, several conditions (e.g. incubation time and heparinase I concentration) were optimized by using the aXa assay for monitoring. Defined UFH/OSCS mixtures incubated in this way showed a concentration-dependent FI increase in the polymer-H assay (λ (em) 330 nm, λ (ex) 510 nm). The sensitivity was unexpectedly high with an LOD/LOQ of 0.5%/0.6% OSCS content in heparin. Further experiments testing UFH/OSCS mixtures in the aXa assay confirmed our hypothesis: OSCS inhibits heparinase I resulting in incomplete heparin degradation and thus an additional FI increase of polymer-H by intact heparin. This two-step microplate fluorescence assay is a sensitive, rapid, and simple method for quantification of OSCS in heparin. In contrast with 1H NMR and CE, neither expensive equipment nor much experience are required. It could be applied not only in the quality control of heparin, but also in clinical practice, to check the applied heparin preparation when a patient suffers any adverse effect.

Assay principle of the two-step fluorescence assay for the quantification of OSCS in heparin. OSCS inhibits heparinase I resulting in incomplete heparin degradation and thus an additional FI increase of polymer-H by intact heparin. This two-step microplate fluorescence assay is a sensitive, rapid, and simple method for quantification of OSCS in heparin. FI = fluorescence intensity






Similar content being viewed by others
References
FDA, U.S. Food and Drug Administration (2009) http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm112669.htm. Accessed 05 March 2010
Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al-Hakim A, Gunay NS, Zhang Z, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R (2008) Nat Biotechnol 26:669–675
Bianchini P, Liverani L, Spelta F, Mascellani G, Parma B (2007) Semin Thromb Hemost 33:496–502
Alban S (2008) Curr Pharm Des 14:1152–1175
Alban S (2008) Hamostaseologie 28:400–420
Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao G, Galcheva-Gargova Z, Al-Hakim A, Bailey GS, Fraser B, Roy S, Rogers-Cotrone T, Buhse L, Whary M, Fox J, Nasr M, Dal Pan GJ, Shriver Z, Langer RS, Venkataraman G, Austen KF, Woodcock J, Sasisekharan R (2008) N Engl J Med 358:2457–2467
N.N. Heparin sodium (2008) European Pharmacopoeia 6.4: Monograph:0333
N.N. Heparin sodium. Stage 2 (2009) US Pharmacopoeia, Rockville http://www.usp.org/hottopics/heparin.html. Accessed March 05, 2010
EDQM. Heparin sodium, Monograph 0333 (2010) http://www.edqm.eu/medias/fichiers/NEW_Heparin_sodium_0820100333.pdf. Accessed June 01, 2010
Pan J, Qian Y, Zhou X, Pazandak A, Frazier SB, Weiser P, Lu H, Zhang L (2010) Nat Biotechnol 28:203–207
Beyer T, Matz M, Brinz D, Radler O, Wolf B, Norwig J, Baumann K, Alban S, Holzgrabe U (2010) Eur J Pharm Sci. doi:10.1016/j.ejps.2010.04.002
Alban S (2010) Pharm Ztg 12:14–22
Volpi N, Maccari F, Linhardt RJ (2009) Anal Biochem 388:140–145
Beyer T, Diehl B, Randel G, Humpfer E, Schafer H, Spraul M, Schollmayer C, Holzgrabe U (2008) J Pharm Biomed Anal 48:13–19
Trehy ML, Reepmeyer JC, Kolinski RE, Westenberger BJ, Buhse LF (2009) J Pharm Biomed Anal 49:670–673
Hu P, Fang L, Chess EK (2009) Anal Chem 81:2332–2343
Norwig J, Beyer T, Brinz D, Holzgrabe U, Diller M, Manns D (2009) Pharmeur Sci Notes 2009:17–24
Alban S, Lühn S (2008) N Engl J Med 359:2732–2734
Wang L, Buchanan S, Meyerhoff ME (2008) Anal Chem 80:9845–9847
Bairstow S, McKee J, Nordhaus M, Johnson R (2009) Anal Biochem 388:317–321
Lühn S, Schrader T, Sun W, Alban S (2010) J Pharm Biomed Anal 52:1–8
Sun W, Bandmann H, Schrader T (2007) Chemistry 13:7701–7707
Maruyama T, Toida T, Imanari T, Yu G, Linhardt RJ (1998) Carbohydr Res 306:35–43
Alban S, Lühn S (2009) Journal of Thrombosis and Haemostasis 7: Abstract PP-TH-193
Viskov C, Bouley E, Hubert P, Martinez C, Herman F, Jeske W, Hoppensteadt D, Walenga JM, Fareed J (2009) Clin Appl Thromb Hemost 15:395–401
Alban S, Schiemann S (2009) Journal of Thrombosis and Haemostasis 7: Abstract PP-WE-506
Basche M, Gustafson DL, Holden SN, O’Bryant CL, Gore L, Witta S, Schultz MK, Morrow M, Levin A, Creese BR, Kangas M, Roberts K, Nguyen T, Davis K, Addison RS, Moore JC, Eckhardt SG (2006) Clin Cancer Res 12:5471–5480
Acknowledgments
We acknowledge Professor Thomas Schrader and Dr Wei Sun for the supply of the polymer-H.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue on Heparin Characterization with Guest Editor Cynthia K. Larive.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00216-010-4318-z
Rights and permissions
About this article
Cite this article
Lühn, S., Schiemann, S. & Alban, S. Simple fluorescence assay for quantification of OSCS in heparin. Anal Bioanal Chem 399, 673–680 (2011). https://doi.org/10.1007/s00216-010-3867-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3867-5