Abstract
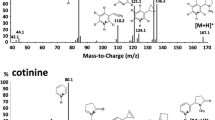
An analytical procedure was developed and validated for the simultaneous identification and quantification of nicotine, cotinine, trans-3′-hydroxycotinine, and norcotinine in 0.5 mL of human oral fluid collected with the Quantisal™ oral fluid collection device. Solid phase extraction and liquid chromatography-tandem mass spectrometry with multiple reaction monitoring were utilized. Endogenous and exogenous interferences were extensively evaluated. Limits of quantification were empirically identified by decreasing analyte concentrations. Linearity was from 1 to 2,000 ng/mL for nicotine and norcotinine, 0.5 to 2,000 ng/mL for trans-3′-hydroxycotinine, and 0.2 to 2,000 ng/mL for cotinine. Correlation coefficients for calibration curves were >0.99 and analytes quantified within ±13% of target at all calibrator concentrations. Suitable analytical recovery (>91%) was achieved with extraction efficiencies >56% and matrix effects <29%. This assay will be applied to the quantification of nicotine and metabolites in oral fluid in a clinical study determining the most appropriate nicotine biomarker concentrations differentiating active, passive, and environmental nicotine exposure.

Similar content being viewed by others
References
Dams R, Choo RE, Lambert WE, Jones H, Huestis MA (2007) Drug Alcohol Depend 87:258–267
Smith FP, Kidwell DA (1996) Forensic Sci Int 83:179–189
Speckl IM, Hallbach J, Guder WG, Meyer LV, Zilker T (1999) Clin Toxicol 37:441–445
U.S. EPA. Respiratory health effects of passive smoking. U.S. Environmental Protection Agency, Office of Research and development, Office of Health and Environmental Assessment, Washington, DC, EPA/600/6-90/006F, 1992
Benowitz NL, Hukkanen J, Jacob P III (2009) Handb Exp Pharmacol 192:29–60
Benowitz NL, Jacob P III (2001) Br J Clin Pharmacol 51:53–59
SRNT Subcommittee on Biochemical Verification (2002) Nicotine Tob Res 4:149–159
Lindkvist B, Wierup N, Sundler F, Borgstrom A (2008) Pancreas 37:288–294
Wagena EJ, de Vos A, Horwith G, van Schayck CP (2008) Nicotine Tob Res 10:213–218
Nishimura H, Furumiya J, Nakanishi A, Hashimoto Y (2009) Leg Med 11:S565–567
Massadeh AM, Gharaibeh AA, Omari KW (2009) J Chromatogr Sci 72:170–177
Gray TR, Shakleya DM, Huestis MA (2008) J Chromatogr B 863:107–114
Pellegrini M, Marchei E, Rossi S, Vagnarelli F, Durgbanshi A, Garcia-Algar O, Vall O, Pichini S (2007) Rapid Commun Mass Spectrom 21:2693–2703
Hoofnagle AN, Laha TJ, Rainey PM, Sadrazadeh SM (2006) Am J Clin Pathol 126:880–887
Xu X, Iba M, Weisel CP (2004) Clin Chem 50:2323–2330
Moyer TP, Charlson JR, Enger RJ, Dale LC, Ebbert JO, Schroeder DR, Hurt RD (2002) Clin Chem 48:1460–1471
Kim I, Huestis MA (2006) J Mass Spectrom 41:815–821
Kim I, Darwin WD, Huestis MA (2005) J Chromatogr B 814:233–240
Kataoka H, Inoue R, Yagi K, Saito K (2009) J Pharma Biomed Anal 49:108–114
Shakleya DM, Huestis MA (2009) J Anal Bioanal Chem 393:1957–1965
Vine MF, Hulka BS, Margolin BH, Truong YK, Hu PC, Schramm MM, Griffith JD, McCann M, Everson RB (1993) Am J Publ Health 83:1335–1338
Binnie V, McHugh S, Macpherson L, Borland B, Noir K, Malik K (2004) Oral Diseases 10:287–293
Bentley MC, Abrar M, Kelk M, Cook J, Phillips K (1999) J Chromatogr B 723:185–194
Byrd GD, Davis RA, Ogden MW (2005) J Chromatogr Sci 43:133–140
Krouwer JS, Rabinowitz R (1984) Clin Chem 30:290–292
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Anal Chem 75:3019–3030
Acknowledgment
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shakleya, D.M., Huestis, M.A. Optimization and validation of a liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of nicotine, cotinine, trans-3′-hydroxycotinine and norcotinine in human oral fluid. Anal Bioanal Chem 395, 2349–2357 (2009). https://doi.org/10.1007/s00216-009-3157-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3157-2