Abstract
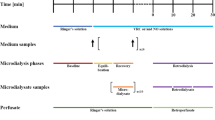
Voriconazole is a very potent antifungal agent used to treat serious fungal infections (candidiasis); it is also the therapy of choice for aspergillosis. After standard dosing, several factors affect exposure of voriconazole, resulting in large variability and demanding further elucidation of drug distribution. For measurements at the site of action, microdialysis is considered to be an outstanding minimally invasive method. For determination of voriconazole in microdialysate and human plasma a new, efficient, reliable, and robust HPLC assay using UV detection at 254 nm has been developed and validated. After simple sample preparation using acetonitrile for plasma and for microdialysate, 20 μL were injected and separated on an RP-18 column. The chromatographic run time was less than 4 min. Overall, the assay showed high precision (CV 93.9 to 99.5%) and accuracy (RE −96.7 to +107%) for both matrices. Of the 36 drug products typically co-administered with voriconazole, none except ambroxol interfered with its peak signal, and this interference was successfully managed. In summary, the method is highly suitable for application in (pre)clinical microdialysis studies, e.g., of critically ill patients with invasive mycoses.

Microdialysis probe situated in the interstitial space fluid containing voriconazole drug molecules (magenta coloured) extracting an important target site representative matrix (microdialysate) [Courtesy of CMA]




Similar content being viewed by others
References
Boucher HW, Groll AH, Chiou CC, Walsh TJ (2004) Drugs 64:1997–2020
Smith J, Safdar N, Knasinski V, Simmons W, Bhavnani SM, Ambrose PG, Andes D (2006) Antimicrob Agents Chemother 50:1570–1572
Quintard H, Papy E, Massias L, Lasocki S, Arnaud P, Desmonts JM, Montravers P (2008) Ther Drug Monit 30:117–119
Pascual A, Nieth V, Calandra T, Bille J, Bolay S, Decosterd LA, Buclin T, Majcherczyk PA, Sanglard D, Marchetti O (2007) Antimicrob Agents Chemother 51:137–143
Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D (2002) Antimicrob Agents Chemother 46:2546–2553
Hyland R, Jones BC, Smith DA (2003) Drug Metab Dispos 31:540–547
Pfizer Inc. Label (2005) LAB-0271–12, Mar
Purkins L, Wood N, Greenhalgh K, Eve MD, Oliver SD, Nichols D (2003) Br J Clin Pharmacol 56(Suppl 1):2–9
Sasongko L, Williams KM, Day RO, McLachlan AJ (2003) Br J Clin Pharmacol 56:551–561
Lutsar I, Roffey S, Troke P (2003) Clin Infect Dis 37:728–732
Theuretzbacher U, Ihle F, Derendorf H (2006) Clin Pharmacokinet 45:649–663
Muller M (2002) Br Med J 324:588–591
Plock N, Kloft C (2005) Eur J Pharm Sci 25:1–24
Araujo BV, Conrado DJ, Palma EC, Dalla Costa T (2007) J Pharm Biomed Anal 15:985–990
Cheng S, Qiu F, Huang J, He J (2007) J Chromatogr Sci 45:409–414
Chhun S, Rey E, Tran A, Lortholary O, Pons G, Jullien V (2007) J Chromatogr B Anal Technol Biomed Life Sci 852:223–228
Gage R, Stopher DA (1998) J Pharm Biomed Anal 17:1449–1453
Keevil BG, Newman S, Lockhart S, Howard SJ, Moore CB, Denning DW (2004) Ther Drug Monit 26:650–657
Langman LJ, Boakye-Agyeman F (2007) Clin Biochem 40:1378–1385
Pehourcq F, Jarry C, Bannwarth B (2004) Biomed Chromatogr 18:719–722
Pennick GJ, Clark M, Sutton DA, Rinaldi MG (2003) Antimicrob Agents Chemother 47:2348–2350
Perea S, Pennick GJ, Modak A, Fothergill AW, Sutton DA, Sheehan DJ, Rinaldi MG (2000) Antimicrob Agents Chemother 44:1209–1213
Roffey SJ, Cole S, Comby P, Gibson D, Jezequel SG, Nedderman AN, Smith DA, Walker DK, Wood N (2003) Drug Metab Dispos 31:731–741
Stopher DA, Gage R (1997) J Chromatogr B Biomed Sci Appl 691:441–448
Wenk M, Droll A, Krahenbuhl S (2006) J Chromatogr B Anal Technol Biomed Life Sci 832:313–316
Food and Drug Administration (FDA) (2001) [Online] http://www.fda.gov/cder/guidance/4252fnl.pdf
International Conference on Harmonisation (ICH) (2005) [Online] http://www.ich.org/LOB/media/MEDIA417.pdf
Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A (2000) Pharm Res 17:1551–1557
Espinel-Ingroff A, Johnson E, Hockey H, Troke P (2008) J Antimicrob Chemother 61:616–620
Sabatelli F, Patel R, Mann PA, Mendrick CA, Norris CC, Hare R, Loebenberg D, Black TA, McNicholas PM (2006) Antimicrob Agents Chemother 50:2009–2015
Acknowledgements
The authors wish to thank Dorothea Frenzel and Lisanne Laetsch for their excellent contribution in conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simmel, F., Soukup, J., Zoerner, A. et al. Development and validation of an efficient HPLC method for quantification of voriconazole in plasma and microdialysate reflecting an important target site. Anal Bioanal Chem 392, 479–488 (2008). https://doi.org/10.1007/s00216-008-2286-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-2286-3