Abstract
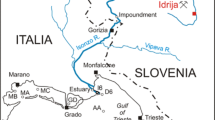
Experiments to determine the mercury methylation potential were performed on sediments from two locations on the river Idrijca (Slovenia), differing in ambient mercury concentrations. The tracer used was the radioactive isotope 197Hg. The benefit of using this tracer is its high specific activity, which enables spikes as low as 0.02 ng Hg2+ g−1 of sample to be used. It was therefore possible to compare the efficiency of the methylation potential experiments over a range of spike concentrations from picogram to microgram levels. The first part of the work aimed to validate the experimental blanks and the second part consisted of several series of incubation experiments on two different river sediments using a range of tracer additions. The results showed high variability in the obtained methylation potentials. Increasing Hg2+ additions gave a decrease in the percentage of the tracer methylated during incubation; in absolute terms, the spikes that spanned four orders of magnitude (0.019–190 pg g−1 of sediment slurry) resulted in MeHg formation between 0.01 and 0.1 ng MeHg g−1 in Podroteja and Kozarska Grapa. Higher spikes resulted in slightly elevated MeHg production (up to a maximum of 0.27 ng g−1). The values of methylation potential were similar in both sediments. The results imply that the experimental determination of mercury methylation potential strongly depends on the experimental setup itself and the amount of tracer added to the system under study. It is therefore recommended to use different concentrations of tracer and perform the experiments in several replicates. The amount of mercury available for methylation in nature is usually very small. Therefore, adding very low amounts of tracer in the methylation potential studies probably gives results that have a higher environmental relevance. It is also suggested to express the results obtained in absolute amounts of MeHg produced and not just as the percentage of the added tracer.









Similar content being viewed by others
References
UNEP (2002) Global mercury assessment. United Nations Environment Programme, Geneva
Horvat M, Kontić B, Kotnik J, Ogrinc N, Jereb V, Logar M, Faganeli J, Rajar R, Širca A, Petkovšek G, Žagar D, Dizdarevič T (2003) Crit Rrev Anal Chem 33:291–296
Munthe J, Bodaly D, Branfireun B, Driscoll C, Gilmour CC, Harris R, Horvat M, Lucotte M, Malm O (2007) Ambio 36(1):33–44
Horvat M, Jereb V, Fajon V, Logar M, Kotnik J, Faganeli J, Hines ME, Bonzongo J-C (2002) Geochem Explor Env A 2:287–296
Kotnik J, Horvat M, Dizdarevič T (2005) Atmos Environ 39(39):7570–7579
Grönlund R, Edner H, Svanberg S, Kotnik J, Horvat M (2005) Atmos Environ 39:4067–4074
Biester H, Gosar M, Müller G (1999) J Geochem Explor 65(3):195–204
Gosar M, Pirc S, Bidovec M (1997) J Geochem Explor 58(2–3):125–131
Kocman D, Horvat M, Kotnik J (2004) J Environ Monitor 6:696–703
Hines ME, Horvat M, Faganeli J, Bonzongo J-C J, Barkay T, Major EB, Scott KJ, Bailey EA, Warwick JJ, Berry Lyons W (2000) Environ Res 83(2):129–139
Hines ME, Faganeli J, Adatto I, Horvat M (2006) Appl Geochem 21:1924–1939
Žižek S, Horvat M, Gibičar D, Fajon V, Toman MJ (2007) Sci Total Environ 377(2–3):407–415
Horvat M, Covelli S, Faganeli J, Logar M, Fajon V, Rajar R, Širca A, Žagar D (1999) Sci Total Environ 237238:43–56
Hintelmann H, Keppel-Jones K, Evans RD (2000) Environ Toxicol Chem 19(9):2204–2211
Rodríguez Martín-Doimeadios RC, Tessier E, Amouroux D, Guyoneaud R, Duran R, Caumette P, Donarda OFX (2004) Mar Chem 90:107–123
Monperrus M, Tessier E, Point D, Vidimova K, Amouroux D, Guyoneaud R, Leynaert A, Grall J, Chauvaud L, Thouzeau G, Donard OFX (2007) Estuar Coast Shelf S 72:485–496
Guimarães JRD, Malm O, Pfeiffer WC (1995) Sci Total Environ 175:151–162
Ribeiro Guevara S, Žižek S, Repinc U, Pérez Catán S, Jaćimović R, Horvat M (2007) Anal Bioanal Chem 387(6):2185–2197
Ramlal PS, Rudd JWM, Hecky RE (1986) Appl Environ Microb 51(1):110–114
Horvat M, Miklavčič V, Pihlar B (1991) Anal Chim Acta 243:71–79
Horvat M, Liang L, Bloom NS (1993) Anal Chim Acta 282:153–168
Kosta L, Byrne AR (1969) Talanta 16:1297–1303
Pérez Catán S, Ribeiro Guevara S, Marvin-DiPasquale M, Magnavacca C, Cohen IM, Arribére M (2007) Appl Radiat Isotopes 65:987–994
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Crit Rev Env Sci Tec 31(3):241–293
Weber JH (1993) Chemosphere 26:2063–2077
Nagase H, Ose O, Sato T, Ishikawa T (1982) Sci Total Environ 25:133–142
Gårdfeldt K, Munthe J, Stromberg D, Lindqvist O (2003) Sci Total Environ 304:127–136
Craig PJ (ed) (1986) Organometallic components in the environment. Principles and reactions. Longman, Harlow
Acknowledgements
This work was implemented in the framework of the bilateral cooperation between Slovenia and Argentina entitled “The production and the use of radiotracers in the biogeochemistry of mercury”, the young researchers programme, the programme P1 – 0143 “Environmental cycling of nutrients and contaminants, mass balance and modelling of environmental processes and risk assessment” and the project L1–7407 “Biological methods as an early warning system in mercury contaminated sites”. The authors also wish to express their gratitude to Dr. Anthony Byrne for his constructive remarks and help with the English language.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Žižek, S., Ribeiro Guevara, S. & Horvat, M. Validation of methodology for determination of the mercury methylation potential in sediments using radiotracers. Anal Bioanal Chem 390, 2115–2122 (2008). https://doi.org/10.1007/s00216-008-1968-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-1968-1