Abstract
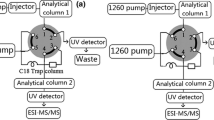
The transfer of a gradient method to an isocratic or multistep gradient method employing stationary phase optimized liquid chromatography facilitated a reduction in analysis time by 50% and significantly improved the mass spectrometric detectability of impurities in synthetic thyroid hormones. Four column segments packed with different stationary phases were combined into a single chromatographic column, which allowed the separation and photometric as well as mass spectrometric detection of thyroid compounds in less than 30 min under isocratic- or step gradient elution conditions with 0.10% acetic acid/acetonitrile. Signal instability and baseline drift during detection by negative electrospray ionization time-of-flight mass spectrometry were minimized by optimizing the spray parameters for each individual elution step. This resulted in improved detectabilities and higher mass spectral quality, especially for low-abundance components in the sample mixture. The method was applied to the separation and detection of the low-abundance impurities formed upon the thermal stressing of a sample of synthetic levothyroxine.








Similar content being viewed by others
Reference
Zhang J, Lazar MA (2000) Annu Rev Physiol 62:439–466
Escobar-Morreale HF, Botella-Carretero JI, Escobar del Rey F, Morreale de Escobar G (2005) J Clin Endocrinol Metab 90:4946–4954
Chalmers JR, Dickson GT, Elks J, Hems BA (1949) J Chem Soc 3424–3433
Hillmann G (1956) Z Naturforsch 11b:419–420
Nahm H, Siedel W (1963) Chem Ber 96:1–9
Salamonczyk GM, Oza VB, Sih CJ (1997) Tetrahedron Lett 38:6965–6968
Andre M, Domanig R, Riemer E, Moser H, Groeppelin A (1996) J Chromatogr A 725:287–294
Gika H, Lämmerhofer M, Papadoyannis I, Lindner W (2004) J Chromatogr B 800:193–201
Kannamkumarath SS, Wuilloud RG, Stalcup A, Caruso JA, Patel H, Sakr A (2004) J Anal Atom Spectrosc 19:107–113
Kirkland JJ, Glajch JL (1983) J Chromatogr 255:27–39
Schoenmakers PJ (1986) Optimiation of chromatographic selectivity. Elsevier, Amsterdam
Zhao J, Carr PW (1999) Anal Chem 71:2623–2632
Dolan JW, Snyder LR, Blanc T, Van Heukelem L (2000) J Chromatogr A 897:37–50
Kromidas S (2006) HPLC made to measure a practical handbook for optimization. Wiley-VCH, Weinheim
Nyiredy SZ (2002) J Chromatogr Sci 40:553–563
Nyiredy SZ, Dallenbach-Toelke K, Sticher O (1989) J Liq Chromatogr 12:95–116
Snyder LR (1978) J Chromatogr Sci 223
Kirkland JJ, Glajch JL (1983) J Chromatogr 255:27–39
Nyiredy SZ, Dallenbach-Toelke K, Sticher O (1988) J Planar Chromatogr 1:336–342
Glajch JL, Gluckman JC, Charikofsky JG, Minor JM, Kirkland JJ (1985) J Chromatogr 318:23–39
Welsch T, Dornberger U, Lerche D (1993) J High Res Chromatogr 16:18–26
Mao Y, Carr PW (2000) Anal Chem 72:2788–2796
Mao Y, Carr PW (2000) Anal Chem 72:110–118
Bonn GK (1985) J Chromatogr 322:411–424
Bischoff K, Nyiredy SZ, Szücs Z (2006) Elements for separating substances by distributing between a stationary and a mobile phase, and method for the production of a separating device. WO/2006/125564; PCT/EP2006/004744
Nyiredy SZ, Szucs Z, Szepesy L (2007) J Chromatogr A 1157:122–130
Nyiredy SZ, Szucs Z, Szepesy L (2006) Chromatographia 63:S3–S9
Kazemifard AG, Moore DE, Aghazadeh A (2001) J Pharm Biomed Anal 25:697–711
Toussaint B, Schimmel H, Klein CL, Wiergowski M, Emons H (2007) J Chromatogr A 1156:236–248
Zhou S, Hamburger M (1996) J Chromatogr A 755:189–204
Dams R, Benijts T, Gunther W, Lambert W, De Leenheer A (2002) Rapid Commun Mass Spectrom 11:1072–1077
Neue UD, O’Gara JE, Mendez A (2006) J Chromatogr A 1127:161–174
Acknowledgements
Financial support by Bischoff Analysentechnik und -geräte GmbH (Leonberg, Germany) and helpful discussions with K. Bischoff and S. Lamotte are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gostomski, I., Braun, R. & Huber, C.G. Detection of low-abundance impurities in synthetic thyroid hormones by stationary phase optimized liquid chromatography–mass spectrometry. Anal Bioanal Chem 391, 279–288 (2008). https://doi.org/10.1007/s00216-008-1920-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-1920-4