Abstract
A coprecipitation method using sample constituents as carrier precipitants was developed that can remove molybdenum, which interferes with the determination of cadmium in grain samples via isotope dilution inductively coupled plasma mass spectrometry (ID-ICPMS). Samples were digested with HNO3, HF, and HClO4, and then purified 6 M sodium hydroxide solution was added to generate colloidal hydrolysis compounds, mainly magnesium hydroxide. Cadmium can be effectively separated from molybdenum because the cadmium forms hydroxides and adsorbs onto and/or is occluded in the colloid, while the molybdenum does not form hydroxides or adsorb onto the hydrolysis colloid. The colloid was separated by centrifugation and then dissolved with 0.2 M HNO3 solution to recover the cadmium. The recovery of Cd achieved using the coprecipitation was >97%, and the removal efficiency of Mo was approximately 99.9%. An extremely low procedural blank (below the detection limit of ICPMS) was achieved by purifying the 6 M sodium hydroxide solution via Mg coprecipitation using Mg(NO3)2 solution. The proposed method was applied to two certified reference materials (NIST SRM 1567a wheat flour and SRM 1568a rice flour) and CCQM-P64 soybean powder. Good analytical results with small uncertainties were obtained for all samples. This method is simple and reliable for the determination of Cd in grain samples by ID-ICPMS.

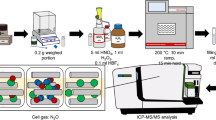
Overview of a coprecipitation method using sample constituents


Similar content being viewed by others
References
Nordberg GF (2004) BioMetals 17:485–489
CODEX Alimentarius Commission (2006) CODEX Alimentarius website. http://www.codexalimentarius.net/web/index_en.jsp. Cited 5th June 2007
Okamoto K (1991) Sci Total Environ 107:29–44
Cubadda F (2004) J AOAC Int 87:173–204
Vassileva E, Quetel CR (2004) Anal Chim Acta 519:79–86
Vassileva E, Quetel CR, Petrov I (2003) Spectrochim Acta B 58:1553–1565
Park CJ, Suh JK (1997) J Anal Atom Spectrom 12:573–577
Klingbeil P, Vogl J, Pritzkow W, Riebe G, Müller J (2001) Anal Chem 73:1881–1888
Fortunato G, Wunderli S (2003) Anal Bioanal Chem 377:111–116
Vogl J (2007) J Anal Atom Spectrom 22:475–492
Inagaki K, Takatsu A, Uchiumi A, Nakama A, Okamoto K (2001) J Anal Atom Spectrom 16:1370–1374
Rodushkin I (1998) Fresenius J Anal Chem 362:541–546
Karunasagar D, Arunachalam J (2001) Anal Chim Acta 441:291–296
Li PC, Jiang SJ (2003) Anal Chim Acta 495:143–150
Jiang SJ, Palmieri MD, Frits JS, Houk RS (1987) Anal Chim Acta 200:559–571
Hwang TJ, Jiang SJ (1997) J Anal Atom Spectrom 12:579–584
Chang CC, Liu HT, Jiang SJ (2003) Anal Chim Acta 493:213–218
Yip YC, Chu HS, Chan KC, Chan KK, Cheung PY, Sham WC (2007) Anal Bioanal Chem 386:1475–1487
Phuong TD, Chuong PV, Khiem DT, Kokot S (1995) Analyst 124:553–560
Wolnik KA, Fricke FL, Capar SG, Baude GL, Meyer MW, Satzger RD, Kuennen RW (1983) J Agric Food Chem 31:1244–1249
Wolnik KA, Fricke FL, Capar SG, Meyer R, Satzger MW, Bonnin D, Gaston E CM (1985) J Agric Food Chem 33:807–811
Isoyama H, Uchida T, Oguchi K, Iida C, Nakagawa G (1990) Anal Sci 6:385–388
Henrion A (1994) Fresenius J Anal Chem 350:657–658
Ellison SLR, Rosslein M, Williams A (2000) EURACHEM/CITAC guide: quantifying uncertainty in analytical measurement, 2nd edn. CITAC Secretariat, Teddington, UK, pp 89–96
Pella PA, Kingston HM, Sleber JR, Feng LY (1983) Anal Chem 55:1193–1194
Yang JY, Yang MH, Lin SM (1985) Anal Chem 57:472–474
Yang JY, Yang MH, Lin SM (1990) Anal Chem 62:146–150
Inagaki K, Haraguchi H (2000) Analyst 125:191–196
Yang XJ, Pin C (2002) Anal Chim Acta 458:375–386
Acknowledgement
This research was supported by the SME Intellectual Foundation Construction Project of the Japanese Ministry of Economy, Trade and Industry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inagaki, K., Narukawa, T., Yarita, T. et al. Determination of cadmium in grains by isotope dilution ICP–MS and coprecipitation using sample constituents as carrier precipitants. Anal Bioanal Chem 389, 691–696 (2007). https://doi.org/10.1007/s00216-007-1396-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1396-7