Abstract
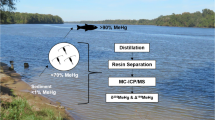
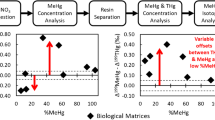
Ultratrace analysis of dissolved MeHg in freshwaters requires both dissociation of MeHg from strong ligands in the sample matrix and preconcentration for detection. Existing solid phase extraction methods generally do not efficiently adsorb MeHg from samples containing high concentrations of natural dissolved organic matter. We demonstrate here that the addition of 10–60 mM thiourea (TU) quantitatively releases MeHg from the dissolved matrix of freshwater samples by forming a more labile complex (MeHgTU+) that quantitatively exchanges MeHg with thiol-functionalized resins at pH∼3.5 during column loading. The contents of these columns were efficiently eluted with acidified TU and MeHg was analyzed by Hg–TU complex ion chromatography with cold-vapor atomic fluorescence spectrometry detection. Routinely more than 90% of MeHg was recovered with good precision (average relative standard deviation of 6%) from natural waters—obtained from pools and saturated sediments of wetlands and from rivers—containing up to 68.7 mg C L−1 dissolved organic matter. With the preconcentration step, the method detection limit of 0.29 pg absolute or 0.007 ng L−1 in 40-mL samples is equivalent to that of the current state-of-the- art as practiced by skilled analysts. MeHg in 20–50-mL samples was completely trapped. On the basis of our knowledge of the chemistry of the process, breakthrough volume should depend on the concentrations of TU and H+. At a TU concentration of 12 mM breakthrough occurred between 50 and 100 mL, but overall adsorption efficiency was still 85% at 100 mL. Formation of artifactual MeHg is minimal; only about 0.7% of ambient MeHg is artifactual as estimated from samples spiked with 4 μg L−1 HgII.







Similar content being viewed by others
References
Watras C, Bloom N (1992) Limnol Oceanogr 37:1313–1318
Bloom N, Colman J, Barber L (1997) Fresenius J Anal Chem 358:371–377
O’Driscoll N, Evans R (2000) Environ Sci Technol 34:4039–4043
Amirbahman A, Reid A, Haines T, Kahl J, Arnold C (2002) Environ Sci Technol 36:690–695
Zhang J, Wang F, House J, Page B (2004) Limnol Oceanogr 49:2276–2286
Hudson R, Gherini S, Watras C, Porcella D (1994) In: Watras C, Huckabee J (eds) Mercury pollution: integration and synthesis. Lewis, Boca Raton, pp 473–523
Watras C, Back R, Halvorsen S, Hudson R, Morrison K, Wente S (1998) Sci Total Environ 219:183–208
Babiarz C, Hurley J, Benoit J, Shafer M, Andren A, Webb D (1998) Biogeochemistry 41:237–257
Ravichandran M (2004) Chemosphere 55:319–331
Horvat M, Lian L, Bloom N (1993) Anal Chim Acta 282:153–168
USEPA (1998) Method 1630: methyl mercury in water by distillation, aqueous ethylation, purge and trap, and CVAFS. US Environmental Protection Agency, Office of Water, Office of Science and Technology, Engineering and Analysis Division, Washington
Law S (1971) Science 174:285–287
Bagheri H, Gholami A (2001) Talanta 55:1141–1150
Salih B, Say R, Denizli A, Genc O, Piskin E (1998) Anal Chim Acta 371:177–185
Emteborg H, Baxter D, Frech W (1993) Analyst 118:1007–1013
Chwastowska J, Rogowska A, Sterlinska E, Dudek J (1999) Talanta 49:837–842
Rudner P, de Torres A, Pavon J, Rojas F (1998) Talanta 46:1095–1105
Lee Y, Mowrer J (1989) Anal Chim Acta 221:259–268
Clarisse O, Hintelmann H (2006) J Environ Monit 8:1242–1247
Cai Y, Jaffe R, Alli A, Jones R (1996) Anal Chim Acta 334:251–259
Munoz J, Gallego M, Valcarcel M (2005) Anal Chim Acta 548:66–72
Falter R, Scholer H (1995) Fresenius J Anal Chem 353:34–38
Blanco R, Villanueva M, Uria J, Sanz-Medel A (2000) Anal Chim Acta 419:137–144
Qvarnstrom J, Tu Q, Frech W, Ludke C (2000) Analyst 125:1193–1197
Castillo A, Roig-Navarro A, Pozo O (2006) Anal Chim Acta 577:18–25
Shade C, Hudson R (2005) Environ Sci Technol 39:4974–4982
Antochshuk V, Jaroniec M (2002) Chem Commun 258–259
Oshita K, Oshima M, Gao Y, Lee K-H, Motomizu S (2002) Anal Sci 18:1121–1125
Antochshuk V, Olkhovyk O, Jaroniec M, Park I-S, Ryoo R (2003) Langmuir 19:3031–3034
Olkhovyk O, Antochshuk V, Jaroniec M (2004) Colloids Surf A 236:69–72
Minagawa K, Takizawa Y, Kifune I (1980) Anal Chim Acta 115:103–110
Fontas C, Hidalgo M, Salvado V, Antico E (2005) Anal Chim Acta 547:255–261
Krishna M, Karunasagar D, Rao S, Arunachalam J (2005) Talanta 68:329–335
Celo V, Ananth R, Scott S, Lean D (2004) Anal Chim Acta 516:171–177
Tekran (2002) Model 2500 CVAFS mercury detector user manual. Tekran, Toronto
Frontier Geosciences (1997) Protocol for sampling waters for MeHg analysis
USGS (1999) National field manual for the collection of water-quality data. US Geological Survey, Reston, chap A5
Robbins J, Gustinis J (1976) Limnol Oceanogr 21:905–909
APHA (1998) Standard methods: for examination of water and wastewater, 20th edn. APHA, Washington
Giambalvo E (1996) A new method for modeling coupled equilibrium and nonequilibrium chemical reactions. Thesis, University of California, Santa Cruz
Dyrssen D, Wedborg M (1991) Water Air Soil Pollut 56:507–519
Cheeseman B, Arnold A, Rabenstein D (1988) J Am Chem Soc 110:6359–6364
Piccolo A, Nardi S, Concheri C (1996) Chemosphere 33:595–602
Haitzer M, Aiken G, Ryan J (2002) Environ Sci Technol 36:3564–3570
USEPA (1995) Method 40 CFR 136 appendix B: definition and procedure for the determination of the method detection limit. US Environmental Protection Agency, Office of Water, Washington
Huang J-H (2005) Anal Chim Acta 532:113–120
NIST (2001) NIST standard reference database 46 v 6.0: NIST critically selected stability constants of metal complexes. NIST, Gaithersburg
USGS (2002) Scientific investigation report 2005-5034: mercury in the Grand Calumet River/Indiana Harbor Canal and Lake Michigan, Lake County, Indiana. US Geological Survey, Reston
Branfireun B (2004) Wetlands 24:207–211
Acknowledgements
The National Great Rivers Research and Education Center in Alton, IL, USA and the Illinois–Indiana Sea Grant College Program funded this work. Thanks are due to C. Shade for instruction in use of the analytical system, currently of Quicksilver Scientific (Lafayette, CO, USA), to W. Wimer for assistance with GCAOC field work, to personnel at the Indiana Dunes National Lakeshore and the Indiana Department of Natural Resources for granting permissions to sample GCAOC wetlands, and to two anonymous reviewers for making helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vermillion, B.R., Hudson, R.J.M. Thiourea catalysis of MeHg ligand exchange between natural dissolved organic matter and a thiol-functionalized resin: a novel method of matrix removal and MeHg preconcentration for ultratrace Hg speciation analysis in freshwaters. Anal Bioanal Chem 388, 341–352 (2007). https://doi.org/10.1007/s00216-007-1207-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1207-1