Abstract
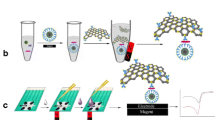
Detecting and enumerating fecal coliforms, especially Escherichia coli, as indicators of fecal contamination, are essential for the quality control of supplied and recreational waters. We have developed a sensitive, inexpensive, and small-volume amperometric detection method for E. coli β-galactosidase by bead-based immunoassay. The technique uses biotin-labeled capture antibodies (Ab) immobilized on paramagnetic microbeads that have been functionalized with streptavidin (bead–Ab). The bead–Ab conjugate captures E. coli from solution. The captured E. coli is incubated in Luria Bertani (LB) broth medium with the added inducer isopropyl β-D-thiogalactopyranoside (IPTG). The induced β-galactosidase converts p-aminophenyl β-D-galactopyranoside (PAPG) into p-aminophenol (PAP), which is measured by amperometry using a gold rotating disc electrode. A good linear correlation (R2=0.989) was obtained between log cfu mL−1 E. coli and the time necessary to product a specific concentration of PAP. Amperometric detection enabled determination of 2×106 cfu mL−1 E. coli within a 30 min incubation period, and the total analysis time was less than 1 h. It was also possible to determine as few as 20 cfu mL−1 E. coli under optimized conditions within 6–7 h. This process may be easily adapted as an automated portable bioanalytical device for the rapid detection of live E. coli.





Similar content being viewed by others
References
Swaminathan B, Feng P (1994) Rapid detection of food-borne pathogenic bacteria. Ann Rev Microbiol 48:401–406
Archer DL, Kvenberg JE (1985) Incidence and cost of food-borne diarrheal disease in United States. J Food Protect 48:887–894
Ford TE (1999) Microbiological safety of drinking water: United States and global perspectives. Environ Health Perspect 107:191–206
MacKenzie WR, Hoxie NJ, Proctor ME, Gradus MS, Blair KA, Peterson DE, Addiss JJ, Fox KR, Rose JB, Davis JP (1994) A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N Engl J Med 331:161–167
Centers for Disease Control and Prevention (1999) Outbreak of Escherichia coli O157:H7 and Campylobacter among attendees of the Washington Country, Fair-New York. Morb Mortal Wkly Rep 48:803–805
Kondro W (2000) E. coli outbreak deaths spark judicial inquiry in Canada. Lancet 355:2058
Thomas JH, Ronkainen-Matsuno NJ, Farrell S, Halsall HB, Heineman WR (2003) Microdrop analysis of a bead-based immunoassay. Microchem J 74:267–276
Altekruse SF, Cohen ML, Sherdlow DL (1997) Emerging foodborne disease. Emerg Infect Dis 3:285–293
Griffin PM, Tauxe RV (1991) The epidemiology of infections caused by Escherichia coli 0157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome, Tauxe. Epidemiol Rev 13:60–98
Jones DL (1999) Potential health risks associated with the persistence of Escherichia coli O157 in agricultural environments. Soil Use Manag 15:76–83
Willshaw GA, Thirlwell J, Jones AP, Parry S, Salmon RL, Hickey M (1994) Vero cytotoxin-producing Escherichia coli O157 in beefburgers linked to an outbreak of diarrhea, haemorrhagic colitis and haemolytic uraemic syndrome in Britain. Lett Appl Microbiol 19:304–307
Rice EW, Allen MJ, Brender DJ, Edberg SC (1991) Assay for beta-glucuronidase in species of the genus Escherichia and its applications for drinking-water analysis. Appl Environ Microbiol 57:592–593
American Public Health Association (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington DC, Parts 9211B, 9211C, 9221B and 9222
Madonna AJ, Cuyk SV, Voorhees KJ (2003) Detection of Escherichia coli using immunomagnetic separation and bacteriophage amplification coupled with matrix laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 17:257–263
Geissler K, Manafi M, Amoros I, Alonso JL (2000) Quantitative determination of total coliforms and Escherichia coli in marine waters with chromogenic and fluorogenic media. J Appl Microbiol 88:280–285
Hattenberger M, Mascher F, Kalcher K, Marth E (2001) Improved method for the fluorimetric detection of β-d-galactosidase in water. Int J Hyg Environ Health 203:281–287
Campbell GR, Prosser J, Glover A, Killham K (2001) Detection of Escherichia coli O157:H7 in soil and water using multiplex PCR. J Appl Microbiol 91:1004–1010
Sun W, Khosravi F, Albrechtsen H, Brovko LY, Griffiths MW (2002) Comparison of ATP and in vivo bioluminescence for assessing the efficiency of immunomagnetic sorbents for live Escherichia coli O157:H7 cells. J Appl Microbiol 92:1021–1027
Prescott AM, Fricker CR (1999) Use of PNA oligonucleotides for the in situ detection of Escherichia coli in water. Mol Cell Probes 13:261–268
Hurt T, Craven RD, Harvey KG, Zhang P, Price E, Fawcett NC, Evans JA (1998) Detection of E. coli O157:H7 with a quartz crystal biosensor and asymmetric PCR. In: Presented at INABIS’98—5th Internet world congress on biomedical sciences at McMaster University, Canada, December 7–16th, 1998
Stender H, Broomer AJ, Oliveira K, Perry-O’Keefe H, Hyldig-Nielsen JJ, Sage A, Coull J (2001) Rapid detection, identification, and enumeration of Escherichia coli cells in municipal water by chemiluminescent in situ hybridization. Appl Environ Microbiol 67:142–147
Yam WC, Lung ML, Ng MH (1992) Evaluation and optimization of a latex agglutination assay for detection of cholera toxin and Escherichia coli heat-labile toxin. J Clin Microbiol 30:2518–2520
Arbault P, Buecher V, Poumerol S, Sorin ML (2000) Study of ELISA method for detection of E. coli O157 in food. Prog Biotechnol 17:359–368
Yu H, Bruno JG (1996) Immunomagnetic-electrochemiluminescent detection of Escherichia coli O157 and Salmonella typhimurium in foods and environmental water samples. Appl Environ Microbiol 62:587–592
Ho JA, Hsiu-Wen H (2003) Procedures for preparing Escherichia coli O157:H7 immunoliposome and its application in liposome immunoassay. Anal Chem 75:4330–4334
Bouvrette P, Luong JHT (1995) Development of a flow injection analysis (FIA) immunosensor for the detection of Escherichia coli. Int J Food Microbiol 27:129–137
Ruan C, Wang H, Li Y (2002) A bienzyme electrochemical biosensor coupled with immunomagnetic separation for rapid detection of Escherichia coli O157:H7 in food samples. Trans ASAE 45:249–255
Neufeld T, Schwartz-Mittelmann A, Biran D, Ron EZ, Rishpon J (2003) Combined phage typing and amperometric detection of released enzymatic activity for the specific identification and quantification of bacteria. Anal Chem 75:580–585
Wijayawardhana CA, Halsall HB, Heineman WR (1999) Micro volume rotating disk electrode (RDE) amperometric detection for a bead-based immunoassay. Anal Chim Acta 399:3–11
Wijayawardhana CA, Wittstock G, Halsall HB, Heineman WR (2000) Electrochemical immunoassay with microscopic immunomagnetic bead domains and scanning electrochemical microscopy. Electroanalysis 12:640–644
Purushothama S, Kradtap S, Wijayawardhana CA, Halsall HB, Heineman WR (2001) Small volume bead assay for ovalbumin with electrochemical detection. Analyst 126:337–341
Ingle JD Jr, Crouch SR (1988) Spectrochemical analysis. Prentice Hall, NJ, USA, pp 173
Perez F, Tryland I, Mascini M, Fiksdal L (2001) Rapid detection of Escherichia coli in water by a culture-based amperometric method. Anal Chim Acta 427:149–154
Mittelmann AS, Ron EZ, Rishpon J (2002) Amperometric quantification of total coliforms and specific detection of Escherichia coli. Anal Chem 74:903–907
Tims TB, Lim DV (2003) Confirmation of viable E. coli O157:H7 by enrichment and PCR after rapid biosensor detection. J Microbiol Meth 55:141–147
Tu SI, Patterson D, Uknalis J, Irwin P (2000) Detection of Escherichia coli O157:H7 using immunomagnetic capture and lucerin–luciferase ATP measurement. Food Res Int 33:375–380
Ertl P, Wagner M, Corton E, Mikkelsen SR (2003) Rapid identification of viable Escherichia coli subspecies with an electrochemical screen-printed biosensor array. Biosens Bioelectron 18:907–916
Perez FG, Mascini M, Tothill IE, Turner APF (1998) Immunomagnetic separation with mediated flow injection analysis amperometric detection of viable Escherichia coli O157. Anal Chem 70:2380–2386
Acknowledgements
The authors would like to thank Professor Brian Kinkle in the Department of Biological Sciences, University of Cincinnati, OH, USA for his counsel and for an E. coli culture. Ismail H. Boyaci would like to thank TUBITAK (The Scientific and Technical Research Council of Turkey) for a fellowship to do research at the University of Cincinnati.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boyacı, İ.H., Aguilar, Z.P., Hossain, M. et al. Amperometric determination of live Escherichia coli using antibody-coated paramagnetic beads. Anal Bioanal Chem 382, 1234–1241 (2005). https://doi.org/10.1007/s00216-005-3263-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-3263-8