Abstract
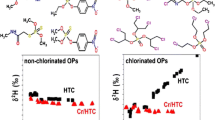
2H/1H isotope ratios of polyhalogenated compounds were determined by elemental analysis and isotope ratio mass spectrometry (EA-IRMS). Initial measurements with standard EA-IRMS equipment, which used high-temperature pyrolysis to convert the organic compounds into hydrogen, did not achieve significant signals for polychlorinated pesticides and related compounds, presumably due to the formation of HCl instead of hydrogen. To reverse this problematic reaction, a chromium reactor was incorporated into the element analyzer system, which scavenged Cl, forming chromium chloride and releasing hydrogen again in the form of H2. The optimized system therefore allowed the δ2H values of polyhalogenated compounds to be determined. A quality assurance program was developed based on several parameters. (i) Each compound was analyzed using a sequence of five injections, where the first measurement was discarded. (ii) Recovery of H (when calculated relative to acetanilide) had to be >90% for all replicates in a sequence. (iii) All δ-values within a sequence had to vary by less than 10‰. (iv) Results had to be reproducible on another day with a different sample scheme. Once this reproducibility had been established, variabilities in the δ2H values of organohalogen standards were investigated using the technique. The highest δ2H value of +75‰ was found for o,p´-DDD, whereas the strongest depletion in deuterium was found for Melipax (–181‰). The most important results for comparable compounds were as follows. DDT-related compounds gave δ2H values of between +59 and +75‰ (technical DDT, o,p´- and p,p´-DDD) or in the range of approximately −1‰, indicative of the different sources/methods of producing this compound. Four HCH isomers from the same supplier showed relatively similar hydrogen isotope distributions, whereas two lindane (γ–HCH) standards from other sources had 39‰ less deuterium. This difference is likely due to different purification steps during the isolation of pure lindane from the technical HCH mixture. An even greater difference was observed between the δ2H values of Toxaphene (US product dating from 1978) and Melipax (product from the former East Germany, dating from 1979), which gave δ2H values of –101‰ and –181‰, respectively, meaning that both products were easily distinguished via δ2H-IRMS. Fractioning of hydrogen isotopes in the atmospheric water cycle was suggested as one reason for the different values. In this theory, the water (which had different δ2H values depending on where it was taken from) was incorporated during the biosynthesis of camphene, which is the natural product used to produce both products. These results indicate that hydrogen isotope-specific analysis can be a valuable tool for tracing the origins of a compound in certain cases.



Similar content being viewed by others
References
Schmidt TC, Zwank L, Elsner M, Berg M, Meckenstock RU, Haderlein SB (2004) Anal Bioanal Chem 378:283–300
Mancini SA, Ulrich AC, Lacrampe-Couloume G, Sleep B, Edwards EA, Sherwood Lollar B (2003) Appl Environ Microbiol 69:191–198
Sewenig S, Bullinger D, Hener U, Mosandl A (2005) J Agric Food Chem 53:838–844
Aguilar-Cisneros BO, Lopez MG, Richling E, Heckel F, Schreier P (2002) J Agric Food Chem 50:7520–7523
Rossmann A (2001) Food Rev Int 17:347–381
Hunkeler D, Aravena R (2000) Appl Environ Microbiol 66:4870–4876
Chu HK, Mahendra S, Song DL, Conrad ME, Alvarez-Cohen L (2004) Environ Sci Technol 38:3126–3130
Hunkeler D, Andersen N, Aravena R, Bernasconi SM, Butler BJ (2001) Environ Sci Technol 35:3462–3467
Vetter W, Gleixner G, Armbruster W, Ruppe S, Stern G, Braekevelt E (2005) Chemosphere 58:235–241
Jarman WA, Hilkert A, Bacon C, Collister JW, Ballschmiter K, Riseborough RW (1998) Environ Sci Technol 32:833–836
Horii Y, Kannan K, Petrick G, Gamo T, Falandysz J, Yamashita N (2005) Environ Sci Technol 39:4206–4212
Midwood AJ, McGaw BA (1999) Anal Commun 36:291–294
Koziet J (1997) J Mass Spectrom 32:103–108
Begley IS, Scrimgeour CM (1997) Anal Chem 69:1530–1535
Kelly SD, Parker LG, Sharman M, Denis MJ (1998) J Mass Spectrom 33:735–738
Vetter W, Gleixner G, Armbruster W (2004) Organohalogen Compd 66:354–357
Kelly SD, Heaton KD, Brereton P (2001) Rapid Comm Mass Spectrom 15:1283–1286
Gehre M, Hoefling R, Kowski P, Strauch G (1996) Anal Chem 68:4414–4417
Morrison J, Brockwell T, Merren T, Fourel F, Phillips AM (2001) Anal Chem 73:3570–3575
WHO (ed) (1989) DDT and its derivatives - environmental aspects (Environ Health Crit 83). WHO, Geneva, ISBN 92–4–154283–7
Breivik K, Pacyna JM, Münch J (1999) Sci Total Environ 239:151–163
C.I.E.L.-Redaktion (ed) (1983) Lindan - Informationen über einen Wirkstoff. Centre International D’etudes du Lindane (Lindan Workshop Hannover), Freiburg
Voldner EC, Li Y-F (1995) Sci Total Environ 160/161:201–210
Vetter W, Oehme M (2001) Toxaphene. Analysis and environmental fate of congeners. In: Paasivirta J (ed) The handbook of environmental chemistry, vol 3, part K: New types of persistent halogenated compounds. Springer, Berlin Heidelberg New York, pp 237–287
Korytár P, van Stee LLP, Leonards PEG, de Boer J, Brinkman UATh (2003) J Chromatogr A 994:179–189
Mook WG (ed) (2001) Environmental isotopes in the hydrological cycle: Principles and applications. Vol I: Introduction: theory, methods, review. IAEA, Vienna
Mullin MD, Pochini CM, McCrindle S, Romkes M, Safe SH, Safe LM (1984) Environ Sci Technol 18:468–476
Vetter W, Luckas B, Haubold W (1991) Chemosphere 23:193–198
Werner RA, Brand WA (2001) Rapid Comm Mass Spectrom 15:501–519
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armbruster, W., Lehnert, K. & Vetter, W. Establishing a chromium-reactor design for measuring δ2H values of solid polyhalogenated compounds using direct elemental analysis and stable isotope ratio mass spectrometry. Anal Bioanal Chem 384, 237–243 (2006). https://doi.org/10.1007/s00216-005-0160-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-0160-0