Abstract
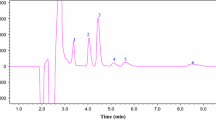
Room temperature ionic liquids (RTIL) are molten salts that are liquids at room temperature. Their liquid state makes them possible candidates as solvents in countercurrent chromatography (CCC), which uses solvents as both the mobile and stationary phases. The study focuses on 1-butyl-3-methylimidazolium hexafluorophosphate (BMIM PF6), an easy to synthesize and purify RTIL whose melting point is −8°C. It is shown that BMIM PF6 behaves like a solvent of significant polarity (comparable with that of ethanol). The ternary phase diagram water–acetonitrile–BMIM PF6 is given, because it was necessary to add acetonitrile to reduce the ionic liquid viscosity. The 40:20:40% w/w water–acetonitrile–BMIM PF6 biphasic liquid system was found to be appropriate as a biphasic liquid system for CCC. Different aromatic solutes, including bases, acids, and neutral compounds, were injected into the CCC column to estimate their distribution constants between the ionic liquid-rich phase and the aqueous phase. The resulting Kil/w constants were compared with the corresponding literature octanol–water partition coefficients, Ko/w. The important drawbacks in the use of RTIL in CCC are clearly pointed out: high viscosity producing pressure build-up, UV absorbance limiting the use of the convenient UV detector, and non-volatility precluding the use of the evaporative light-scattering detector for continuous detection.





Similar content being viewed by others
References
Berthod A (2002) Countercurrent chromatography. The support-free liquid stationary phase. In: Barcelo D (ed) Comprehensive analytical chemistry, vol 38. Elsevier, The Netherlands
Craig LC, Post O (1949) Apparatus for countercurrent distribution. Anal Chem 21:500–504
Rice NM, Irving HMNH, Leonard MA (1993) Nomenclature for liquid-liquid distribution (solvent extraction). Pure Appl Chem 65:2373–2396
Mandava NB, Ito Y (1985) Countercurrent chromatography, theory and practice. Chromatographic Science Series, vol 44. Marcel Dekker, New York
Conway WD (1990) Countercurrent chromatography, apparatus, theory and applications. VCH Publishers, Weinheim
Poole CF (2004) Chromatographic and spectroscopic methods for the determination of solvent properties of room temperature ionic liquids. J Chromatogr A 1039:377–399
Welton T (1999) Room temperature ionic liquid. Solvents for synthesis and catalysis. Chem Rev 99:2071–2083
Earle MJ, Seddon KR (2000) Ionic liquids. Green solvents for the future. Pure Appl Chem 72:1391–1398
Chauvin Y, Olivier-Bourbigou H (1995) Nonaqueous ionic liquids as reaction solvents, Chemtech 25:26–30
Ngo HL, LeCompte K, Hargens L, McEwen AB (2000) Thermal properties of imidazolium ionic liquid, Thermochim Acta 357:97–102
Carda-Broch S, Berthod A, Armstrong DW (2003) Solvent properties of 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid. Anal Bioanal Chem 375:191–196
Berthod A, Carda-Broch S (2003) A new class of solvents for CCC: the room temperature ionic liquids. J Liq Chromatogr Rel Technol 26:1493–1508
Ito Y (1999) Coil planet centrifuge for high speed countercurrent chromatography. In: Menet JM, Thiebaut D (eds) Countercurrent chromatography. Chromatographic Science Series, vol 82. Marcel Dekker, New York
Berthod A, Bully M (1991) High speed countercurrent chromatography used for alkylbenzene liquid-liquid partition coefficient determination, Anal Chem 63:2508–2512
Du Q, Wu C, Qian G, Wu P, Ito Y (1999) Relationship between the flow-rate of the mobile phase and retention of the stationary phase in CCC. J Chromatogr A 835:231–235
Menges RA, Bertrand GL, Armstrong DW (1990) Direct measurement of octanol-water partition coefficients using centrifugal partition chromatography with a back-flushing technique. J Liq Chromatogr 13:3061–3077
Gluck SJ, Martin EJ (1990) Assessment of centrifugal partition chromatography for determination of octanol-water partition coefficients. J Liq Chromatogr 13:2529–2542
Berthod A (1995) Liquid–liquid partition coefficients. The particular case of octanol-water coefficients. In: Foucault A (ed) Centrifugal partition chromatography. Chromatographic Science Series, vol 68, chap 7. Marcel Dekker, New York, pp 167–197
ClogP Computer Program, version 4.01. BioByte Corp., Claremont. http://www.daylight.com/daycgi/clogp
Acknowledgements
SCB thanks the European Community for the Marie Curie fellowship HPMF-CT-2000-00440 (Human Potential) and the foundation Caixa Castelló Bancaixa, both of which made this work possible. AB thanks the French Centre National de la Recherche Scientifique UMR–5180 for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berthod, A., Carda-Broch, S. Use of the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate in countercurrent chromatography. Anal Bioanal Chem 380, 168–177 (2004). https://doi.org/10.1007/s00216-004-2717-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-004-2717-8