Abstract
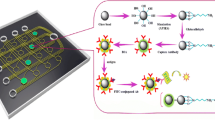
The suitability of a microelectrode as the detector for a small-volume, bead-based enzyme-labeled immunoassay for later use in a microfluidic device was investigated. The microelectrode helps to overcome consumption of the electroactive species by the electrode (depletion) that is encountered with macroelectrodes such as the rotating disk electrode (RDE) and allows the volume of the detection cell to be reduced. Microelectrodes also allow the chemical reactions to be monitored in real time due to the electrodes’ close proximity to the assay site. A bead-based sandwich immunoassay for mouse IgG was developed with alkaline phosphatase (AP) as the enzyme label, p-aminophenyl phosphate (PAPP) as the enzyme substrate, and microelectrode detection. The diffusion coefficient of the product of enzymatic hydrolysis, p-aminophenol (PAP), was determined to be 7.2±0.9×10−6 cm2 s−1. The detection limits were determined for free (0.52 ng mL−1) and bead-bound AP (10 ng mL−1). The number of binding sites for AP per bead was calculated to be 9.6×104 molecules/bead, and under saturation conditions the minimum detectable number of beads was 2500. Lower detection limits could be achieved with the microelectrode than the RDE while maintaining similar reproducibility. The microelectrode also made it possible to work with lower sample volumes (down to 10 μL) than with the RDE (minimum volume of 40 μL). Depletion of PAP was not observed with the microelectrode. The results obtained here with a microelectrode showed great promise for later use of microelectrodes in microfluidic devices with limited sample volumes. RDE detection cannot be used in a microfluidic system due to its complex set-up that includes a motor for rotation.









Similar content being viewed by others
Abbreviations
- Ab:
-
Antibody
- Ag:
-
Antigen
- AP:
-
Alkaline phosphatase
- NSA:
-
Nonspecific adsorption
- PAP:
-
p-aminophenol
- PAPP:
-
p-aminophenyl phosphate
- RDE:
-
Rotating disk electrode
- NSA:
-
Nonspecific adsorption
- PBS:
-
Phosphate-buffered saline
References
Matysik F-M, Nyholm L, Markides KE (1999) Comparison of μm and mm sized disk electrodes for end-column electrochemical detection in capillary electrophoresis. Fresenius J Anal Chem 363:231–235
Ueno K, Kitamura N (2003) A spectroelectrochemical study on perylene cation radical in polymer microchannel–microelectrode chips. Analyst 128:1401–1405
Reichle C, Schnelle T, Muller T, Leya T, Fuhr G (2000) A new microsystem for automated electrorotation measurements using laser tweezers. Biochim Biophys Acta 1459:218–229
Vandaveer WR, Pasas SA, Martin SR, Lunte SM (2002) Recent developments in amperometric detection for microchip capillary electrophoresis. Electrophoresis 2002:3667–3677
Rossier JS, Girault HH (2001) Enzyme-linked immunosorbent assay on a microchip with electrochemical detection. Lab Chip 1:153–157
Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ (1988) Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience 25(2):513–523
Satoh H, Okabe S, Norimatsu N, Watanabe Y (2000) Significance of substrate C/N ratio on structure and activity of nitrifying biofilms determined by in situ hybridization and the use of microelectrodes. Water Sci Technol 41(4–5):317–321
Ouvry A, Cachon R, Divies C (2001) Application of microelectrode technique to measure pH and oxidoreduction potential gradients in gelled systems as model food. Biotech Lett 23:1373–1377
Abe T, Lau Y, Ewing A (1992) Characterization of glucose microsensors for intracellular measurements. Anal Chem 64:2160–2163
Kennedy RT, Huang L, Atkinson MA, Dush P (1993) Amperometric monitoring of chemical secretions from individual pancreatic β-cells. Anal Chem 65:1882–1887
Ewing AG, Dayton MA, Wightman RM (1981) Pulse voltammetry with microvoltammetric electrodes. Anal Chem 53:1842–1847
Kissinger PT, Heineman WR (1996) Laboratory Techniques in Electroanalytical Chemistry, 2nd edn. Marcel Dekker, New York
Clark RA, Zerby SE, Ewing AG (1998) In: Bard AJ, Rubinstein I (eds) Electroanalytical Chemistry, vol 20; Marcel Dekker, New York, pp 227–294
Hu Z, Heineman WR (2000) Oxidation-state speciation of [ReII (DMPE)3 ]2+ by voltammetry with a chemically modified microelectrode. Anal Chem 72:2395–2400
Masson M, Liu Z, Haruyama T, Kobatake E, Ikariyama Y, Aizawa M (1995) Immunosensing with amperometric detection, using galactosidase as label and p-aminophenyl-β-D-galactopyranoside as substrate. Anal Chim Acta 304:353–359
Yao H, Halsall HB, Heineman WR, Jenkins SH (1995) Electrochemical dehydrogenase-based homogeneous assays in whole blood. Clin Chem 41(4):591–598
Wijayawardhana CA, Purushothama S, Cousino MA, Halsall HB, Heineman WR (1999) Rotating disk electrode amperometric detection for a bead-based immunoassay. J Electroanal Chem 468:2–8
Wijayawardhana CA, Halsall HB, Heineman WR (1999) Micro volume rotating disk electrode (RDE) amperometric detection for a bead-based immunoassay. Anal Chim Acta 399:3–11
Purushothama S, Kradtap S, Wijayawardhana CA, Halsall HB, Heineman WR (2001) Small volume bead assay for ovalbumin with electrochemical detection. Analyst 126:337–341
Kradtap S, Wijayawardhana CA, Schlueter KT, Halsall HB, Heineman WR (2001) Bugbead: an artificial microorganism model used as a harmless simulant for pathogenic microorganisms. Anal Chim Acta 444:13–26
Jenkins SH, Halsall HB, Heineman WR (1988) Extending the detection limit of solid-phase electrochemical enzyme immunoassay to the attomole level. Anal Biochem 168:291–294
Halsall HB, Heineman WR, Jenkins SH (1988) Capillary immunoassay with electrochemical detection. Clin Chem 34:1701–1702
Thompson RQ, Barone III GC, Halsall HB, Heineman WR (1991) Comparison of methods for following alkaline phosphatase catalysis: spectrophotometric versus amperometric detection. Anal Biochem 192:90–95
Tang HT, Lunte GE, Halsall HB, Heineman WR (1988) p-Aminophenyl phosphate: an improved substrate for electrochemical enzyme immunoassay. Anal Chim Acta 214:187–195
Rosen I, Rishpon J (1989) Alkaline phosphatase as a label for a heterogeneous immunoelectrochemical sensor. J Electroanal Chem 258:27–39
Yu Z, Xu YI, Ip MPC (1994) An ultra-sensitive electrochemical enzyme immunoassay for thyroid stimulating hormone in human serum. J Pharm Biomed Anal 12(6):787–793
Kronkvist K, Lovgren Ulf, Edholm LE, Johansson G (1993) Determination of drugs in biosamples at picomolar concentrations using competitive ELISA with electrochemical detection: application to steroids. J Pharm Biomed Anal 11(6):459–467
Niwa O, Xu Y, Halsall HB, Heineman WR (1993) Small-volume voltammetric detection of 4-aminophenol with interdigitated array electrodes and its application to electrochemical enzyme immunoassay. Anal Chem 65(11):1559–1563
Aguilar ZP, Vaveer WR, Fritsch I (2002) Self-contained microelectrochemical immunoassay for small volumes using mouse IgG as a model system. Anal Chem 74(14):3321–3329
Moore EJ, Pravda M, Kreuzer MP, Guilbault GG (2003) Comparative study of 4-aminophenyl phosphate and ascorbic acid 2-phosphate, as substrates for alkaline phosphatase based amperometric immunosensor. Anal Lett 36(2):303–315
Stulik K, Amatore C, Holub K, Marecek V, Kutner W (2000) Microelectrodes. Definitions, characterization, and applications. Pure Appl Chem 72(8):1483–1492
Oldham KB, Myland JC (1994) Fundamentals of electrochemical science. Academic, San Diego
Robinson D, Anderson JE, Lin J (1990) Measurement of diffusion coefficients of some indoles and ascorbic acid by flow injection analysis. J Phys Chem 94:1003–1005
Snead WK, Remick AE (1957) Studies on oxidation-reduction mechanism. II. The anodic oxidation of p-aminophenol. J Am Chem Soc 79:6121–6127
Cornish-Bowden A (1979) Fundamentals of enzyme kinetics, 2nd edn. Butterworth, London
Aslam M, Dent A (1998) Bioconjugation. Macmillan Reference Ltd
Acknowledgements
This work was supported by the Doctoral Investment Award, Ohio Board of Regents. S.F. acknowledges the Stecker Fellowship and the University Research Council Fellowship sponsored by the University of Cincinnati.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farrell, S., Ronkainen-Matsuno, N.J., Halsall, H.B. et al. Bead-based immunoassays with microelectrode detection. Anal Bioanal Chem 379, 358–367 (2004). https://doi.org/10.1007/s00216-004-2632-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-004-2632-z