Abstract
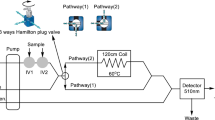
The present work reports for the first time a simple and rapid method for the spectrofluorimetric determination of lisinopril (LSP) in pharmaceutical formulations using sequential injection analysis (SIA). The method is based on reaction of LSP with o-phthalaldehyde (OPA) in the presence of 2-mercaptoethanol (borate buffer medium, pH=10.6). The emission of the derivative is monitored at 455 nm upon excitation at 346 nm. The various chemical and physical conditions that affected the reaction were studied. The calibration curve was linear in the range 0.3–10.0 mg L−1 LSP, at a sampling rate of 60 injections h−1. Consumption of OPA reagent was significantly reduced compared with conventional flow injection (FI) systems, because only 50 μL of OPA was consumed per run. The method was found to be adequately precise (s r=2% at 5 mg L−1 LSP, n=10) and the 3σ detection limit was 0.1 mg L−1. The method was successfully applied to the analysis of two pharmaceutical formulations containing LSP. The results obtained were in good agreement with those obtained by use of high-performance liquid chromatography (HPLC), because the mean relative error, e r, was <1.8%.




Similar content being viewed by others
References
United States Pharmacopeia (1995) XXIII US Pharmacopeial Convention, Rockville, MD, p 895
The official Lisinopril web site, http://www.lisinopril.com
Paraskevas G, Atta-Politou J, Koupparis M (2002) J Pharm Biomed Anal 29:865–872
El-Yazbi FA, Abdine HH, Shaalan RA (1999) J Pharm Biomed Anal 19:819–827
El-Gindy A, Ashour A, Abdel-Fattah L, Shabana MM (2001) J Pharm Biomed Anal 25:913–922
Abdel Razak O, Belal SF, Bedair MM, Barakat NS, Haggag RS (2003) J Pharm Biomed Anal 31:701–711
Ozer D, Senel H (1999) J Pharm Biomed Anal 21:691–695
Wong YC, Charles BG (1995) J Chromatogr B 673:306–310
Tsakalof A, Bairachtari K, Georgarakis M (2003) J Chromatogr B 783:425–432
Hillaert S, van de Bossche W (2000) J Chromatogr A 895:33–42
Hillaert S, de Grauwe K, van de Bossche W (2001) J Chromatogr A 924:439–449
Abdel Razak O, Belal SF, Bedair MM, Haggag RS (2003) Talanta 59:1061–1069
Yuan AS, Gilbert JD (1996) J Pharm Biomed Anal 14:773–778
Ruzicka J, Marshall GD (1990) Anal Chim Acta 237:329–343
Aktas ES, Ersoy L, Sagirli O (2003) Il Farmaco 58:165–168
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zacharis, C.K., Tzanavaras, P.D., Themelis, D.G. et al. Rapid spectrofluorimetric determination of lisinopril in pharmaceutical tablets using sequential injection analysis. Anal Bioanal Chem 379, 759–763 (2004). https://doi.org/10.1007/s00216-004-2530-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-004-2530-4