Abstract
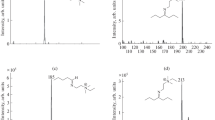
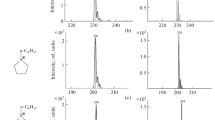
Electrospray ionization mass spectrometry (ESI-MS) coupled to a microreactor is an excellent tool for the investigation of reactions in solution. Here, we report the first results of our investigations into preparatively interesting electron-transfer-initiated chain reactions in solution which proceed via radical cations as reactive intermediates. The tris(p-bromophenyl)aminium hexachloroantimonate (1)-mediated [2+2] cycloaddition of trans-anethole (2) to give 1,2-bis(4-methoxyphenyl)-3,4-dimethylcyclobutane (3) was investigated. The reaction proceeds as a radical cation chain reaction via transient intermediates 2 ●+ and 3 ●+ that could be detected and characterized unambiguously directly in the reacting solution by ESI-MS/MS. The identity of the intermediates was confirmed by comparison with authentic MS/MS spectra of 2 ●+ and 3 ●+ obtained by atmospheric pressure chemical ionization mass spectrometry (APCI-MS). In addition, substrate and product can be monitored easily in the reacting solution by APCI-MS.











Similar content being viewed by others
References
Gordy W (1980) Theory and applications of electron spin resonance. Wiley, New York
Kochi JK (1973) Free radicals. Wiley, New York
Fossey J, Lefort D, Sorba J (1995) Free radicals in organic chemistry. Wiley, New York, pp 5–18
Harrison AG (1992) Chemical ionization mass spectrometry, 2nd edn. CRC, Boca Raton, pp 53–55
Lehmann WD (1996) Massenspektrometrie in der Biochemie. Spektrum, Heidelberg, pp 48–52
Cole RB (ed) (1997) Electrospray ionization mass spectrometry—fundamentals, instrumentation and applications. Wiley, New York
Griep-Raming J, Meyer S, Bruhn T, Metzger JO (2002) Angew Chem 114:2863–2866; Angew Chem Int Ed 41:2738–2742
Schmidt W, Steckhan E (1980) Chem Ber 113:577–585
Balzani V (2001) Electron transfer in chemistry. In: Mattay J (ed) Organic molecules, Vol. II, Part l. Wiley, Weinheim; Bauld NL, Gao D (eds) (2001) The electron-transfer chemistry of carbon–carbon multiple bonds. Wiley, Weinheim, pp 133–205
Bauld NL, Pabon R (1983) J Am Chem Soc 105:633–634
Bauld NL, Bellville DJ, Pabon R, Chelsky R, Green G (1983) J Am Chem Soc 105:2378–2382
Lorenz KT, Bauld NL (1987) J Am Chem Soc 109:1157–1160
Lewis FD, Kojima M (1988) J Am Chem Soc 110:8664–8670
Brede O, David F, Steenken S (1995) J Chem Soc Perkin Trans 2:23–32
Tojo S, Toki S, Taramuku S (1991) J Org Chem 56:6241–6243
Eckert G, Goez M (1994) J Am Chem Soc 116:11999–12009
Goez M, Eckert G (1996) J Am Chem Soc 118:140–154
Roth HD, Weng H, Zhon D, Lakkaraja PS (1997) Acta Chem Scand 51:626–635
Demaille C, Bard AJ (1999) Acta Chem Scand 53:842–848
Meyer S, Koch R, Metzger JO (2003) Angew Chem 115:4848–4851; Angew Chem Int Ed 42:4700–4703
The unit Thomson (Th) was proposed by Cooks RG, Rockwood AL (1991) Rapid Commun Mass Spectrom 5:93
Budzikiewicz H (1998) Mass spectrometry. Wiley, Weinheim
Kuck D, Bruder A, Ramana DV (1997) Int J Mass Spectrom Ion Processes 167/168:55–68
Groenewold GS, Chess EK, Gross ML (1984) J Am Chem Soc 106:539–543
Bellville DJ, Bauld NL (1986) Tetrahedron 42:6167–6173
Belleville DJ, Bauld NL (1982) J Am Chem Soc 104:5700–5702
Jungwirth P, Bally T (1993) J Am Chem Soc 115:5783–5789
Acknowledgements
We thank the German Research Association (DFG) and the Heinz Neumüller Foundation Oldenburg for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Part of this work was presented at the 36th Conference of the German Society for Mass Spectrometry (DGMS).
Rights and permissions
About this article
Cite this article
Meyer, S., Metzger, J.O. Use of electrospray ionization mass spectrometry for the investigation of radical cation chain reactions in solution: detection of transient radical cations. Anal Bioanal Chem 377, 1108–1114 (2003). https://doi.org/10.1007/s00216-003-2230-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-003-2230-5