Abstract
Human hair shavings were characterized as a sorbent for trace metals. At pH 7.0 metal sorption follows the order Pb(II)>Cd(II)>Cr(VI)>Fe(III)>Cu(II)>Ni(II)>Mn(VI). Metal recovery is quantitative for Pb and Cd after 30 min of equilibration. Recovery of other metals is less quantitative and varies with pH. For example, while Cu is best recovered at pH 5, Ni and Mn are sorbed optimally in the basic pH region. Sorbed metals can be washed off the sorbent with 0.5 mol L–1 strong mineral acids or more completely with 0.1 mol L–1 ethylenediaminetetraacetic acid (EDTA). Typical sorption isotherms were obtained for Cd and Pb with sorption capacities of 39 and 26 μmol g–1, respectively.
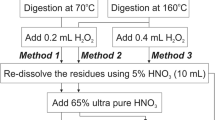
Hair sorbent was used for 40-fold pre-concentration of Cd and Pb from treated wastewater samples followed by flame atomic absorption spectroscopic (FAAS) determination. Comparison of the data obtained for lead and cadmium by the proposed pre-concentration method with that by graphite furnace atomic absorption spectroscopy (GFAAS) showed 79 to 86% recovery and comparable analytical precision. Common cations and anions at the levels normally present in natural water do not interfere in the proposed pre-concentration-FAAS method.



Similar content being viewed by others
References
Minczewski J, Chewastowska J, Dybczynski R (1982) In: Chalmer RA, Masson MR (Eds) Separation and preconcentration methods in inorganic trace analysis. Ellis Horwood, Chichester, UK
Liu Y, Ingle JD Jr (1989) Anal Chem 61:520–524
Bysouth SR, Tyson JF, Stockwell PB (1988) Anal Chim Acta 214:329–337
Purohit R, Devi S (1992) Anal Chim Acta 259:53–60
Rodriguez D, Fernandez P, Perez-Conde C, Gutierrez A, Camara C (1994) Fresenius J Anal Chem 349:442–446
Colognesi M, Abollino O, Aceto M, Sarzanini C, Mentasti E (1997) Talanta 44:867–875
Ke CB, Lin KC (1999) Anal Chem 71:1561–1567
Yan XP, Sperling M, Welz B (1999) Anal Chem 71:4216–4222
Champe PC, Harvey RA (1994) Lippincott's illustrated reviews: biochemistry, 2nd edn. Lippincott, Philadelphia, p 45
Howard M, Jurbergs HA, Holcombe JA (1998) Anal Chem 70:1604–1609
Sverin K, Bergs R, Beck W (1998) Angew Chem Int Ed 37:1634–1654
Bottari E, Festa MR (1997) Talanta 44:1705–1718
Mendieta J, Diaz-Cruz MS, Monjonell A, Tauler R, Esteban M (1999) Anal Chim Acta, 390:15–25
Beveridge TJ, Hughes MN, Lee H, Leung KT, Poole RK, Savvaidis I, Silver S, Trevors JT (1997) Adv Microb Physiol 38:177–243
Bontidean I, Berggren C, Johansson G, Csoregi E, Mattiasson B, Lloyd JR, Jackman KJ, Brown NL (1998) Anal Chem 70:4162–4169
Kadokura S, Miyamoto T, Ito H, Inagaki H (1982) Polymer J 14:121–126
Ishikawa S, Suyamak (1998) Appl Biochem Biotechnol 72:719–728
Ho YS, Wase DAJ, Foster CF (1996) Environ Technol 17:71–77
Nakajima A, Yasuda M, Yokohama H, Ohya-Nishiguki H, Kamada H (2001) World J Microbiol 17:343–347
El-Enany AE, Issa AA (2000) Environ Toxicol Pharmacol 17:71–77
Wasiak W, Ciszewska W, Cisewski A (1996) Anal Chim Acta 335:201–207
Sweileh JA (1999) Al-Manarah 4:173–188
Sweileh JA (2000) Microchem J 65:87–95
Sweileh JA (2001) Anal Chim Acta 448:151–156
Miessler GL, Tarr DA (1991) Inorganic Chemistry. Prentice Hall, Englewood Cliffs, NJ, chap 6
Dias Filho NL, Polito WL, Gushikem Y (1997) Talanta 45:1031–1036
Rio-Segade S, Perez-Cid B, Bendicho C, (1995) Fresenius J Anal Chem 351:798–799
Jain VK, Sait SS, Shrivastav P, Agrawal YK (1995) Talanta 42:397–404
Sweileh JA, Al-Namimi IS, Abulfatih HA, Al-Thani RF, Kardousha AM, Elhaj EA (2002) Qatar Univ Sci J (in press)
Sweileh JA, Lucyk D, Kratochvil B, Cantwell FF (1987) Anal Chem 59:586–592
Acknowledgements
This work was conducted in part in Al-Albayt University laboratories. The technical help of Muhannad Musaad is highly appreciated. Mr Adel Mostafa did the graphite furnace atomic absorption analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sweileh, J.A. Sorption of trace metals on human hair and application for cadmium and lead pre-concentration with flame atomic absorption determination. Anal Bioanal Chem 375, 450–455 (2003). https://doi.org/10.1007/s00216-002-1686-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-002-1686-z