Abstract.
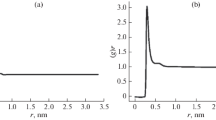
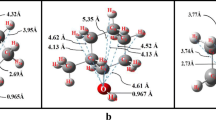
Monte Carlo simulations have been carried out for 2-methoxyethanol in an isothermal–isobaric ensemble (NPT) at 298.15 K and 1 atm pressure. The optimized potential for liquid simulation force field parameters has been used for modeling 2-methoxyethanol and the TIP4P model for water. Intramolecular rotations are described by an analytical potential function fitted to ab initio energies. It has been shown that the water molecules can form hydrogen bonds between adjacent O atoms of CH3OCH2CH2OH in aqueous media. The self-association of 2-methoxyethanol in aqueous media has been studied by statistical perturbation theory.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 9 October 2000 / Accepted: 5 January 2001 / Published online: 3 May 2001
Rights and permissions
About this article
Cite this article
Tafazzoli, M., Jalili, S. Study of association of 2-methoxyethanol in the aqueous phase. Theor Chem Acc 106, 194–198 (2001). https://doi.org/10.1007/s002140100262
Issue Date:
DOI: https://doi.org/10.1007/s002140100262