Abstract.
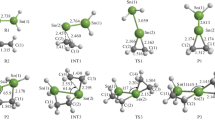
Ethylene insertion into the Sm–C bond of H2SiCp2SmCH3, a model reaction of an olefin polymerization propagation step, has been studied by ab initio molecular orbital methods. The small electronegativity of the Sm atom makes the Sm–C bond ionic, the methyl group being negatively charged by −0.75. The reaction passes through a loose ethylene complex with a binding energy of 15 kcal/mol and then a tight four-centered transition state with an agostic interaction between the Sm atom and one of the methyl CH bonds. A small activation energy of 14 kcal/mol is required to pass through this transition state, indicating that this is an easy reaction. Compared with the reactions with group 4 cationic silylene-bridged metallocenes the activation energy is higher and the reaction is less exothermic. The origin of these differences is discussed. The results of molecular mechanics calculations on regio- and stereoselectivities in the insertion reaction of propylene are also reported.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 13 July 1998 / Accepted: 28 August 1998 / Published online: 2 November 1998
Rights and permissions
About this article
Cite this article
Koga, N. Ab initio MO study of ethylene insertion into the Sm–C bond of H2SiCp2SmCH3. Theor Chem Acc 102, 285–292 (1999). https://doi.org/10.1007/s002140050500
Issue Date:
DOI: https://doi.org/10.1007/s002140050500