Abstract.
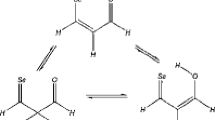
The conformational stabilities of the α- and β-substituted enamines and vinyl ethers were predicted by orbital phase theory and confirmed by ab initio molecular orbital calculations. Cyclic interaction significantly occurs among the nonbonding orbital n Y for the lone pair on the hetero atom Y (N in the enamines or O in the ethers), the π and π* orbitals of the CC bond, and the σC-H or σ*C-X orbitals on the substituent CH2X. The cyclic -n Y-π-σC-H-π*- interaction is favored by the orbital phase continuity in the α-substituted molecules, while the cyclic -n Y-π-σ*C-X-π*- interaction is favored in the β-substituted molecules. The most stable conformation was then predicted to be synperiplanar or (pseudo)equatorial in the α-substituted molecules and anticlinical or (pseudo)axial in the β-substituted molecules.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 8 May 1998 / Accepted: 30 July 1998 / Published online: 16 November 1998
Rights and permissions
About this article
Cite this article
Inagaki, S., Ohashi, S. Orbital phase control of the conformations of α- and β-substituted enamines and vinyl ethers. Theor Chem Acc 102, 65–71 (1999). https://doi.org/10.1007/s002140050473
Issue Date:
DOI: https://doi.org/10.1007/s002140050473